Phospholipid chitosan drug delivery system, preparation method and uses thereof
A delivery system, chitosan technology, applied in the digestive system, nano-drugs, drug combinations, etc., can solve the problems of drug leakage, inability to obtain encapsulation rate, and encapsulation rate of only 52.4±2.4%
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1. Preparation of baicalein-lipid complex
[0055] (1) Preparation of baicalein lipid complex I (baicalein: soybean lecithin (w / w)=1:3.5)
[0056] Take 1g of baicalein, 3.5g of soybean lecithin, add 40mL of tetrahydrofuran, compound at 40°C for 1 hour, remove the solvent by rotary evaporation, and dry in vacuum for more than 12 hours (20-30°C) to obtain baicalein lipid complex I, airtight Pack and store in the refrigerator.
[0057] (2) Preparation of baicalein lipid complex II (baicalein: soybean lecithin (w / w)=1:6)
[0058] Take 0.5g of baicalein and 3.0g of soybean lecithin, add 40mL of ethyl acetate, compound at 40°C for 1 hour, remove the solvent by rotary evaporation, and dry in vacuum for more than 12 hours (20-30°C) to obtain the baicalein lipid complex II, airtightly packaged and stored in the refrigerator.
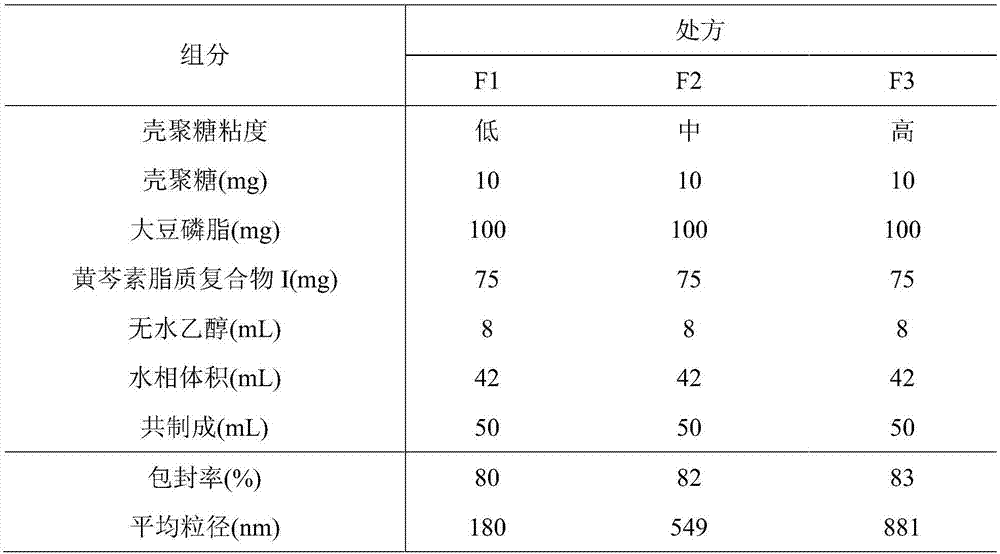
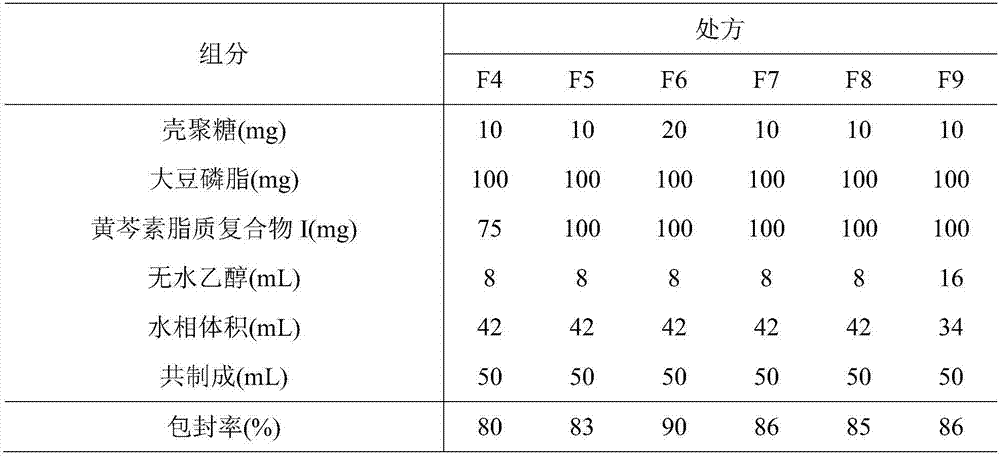
[0059] 2. Preparation of baicalein lipoplex I-phospholipid / chitosan drug delivery system
[0060] (1) Dissolve low, medium and high viscosity chitosan...
Embodiment 2
[0072] 1. Preparation of paclitaxel lipoplex
[0073] (1) Preparation of paclitaxel lipoplex I (paclitaxel: egg yolk phospholipid (w / w)=1:6)
[0074] Take paclitaxel 0.5g, egg yolk phospholipid 3.0g, add tetrahydrofuran 100mL, compound at 40°C for 1 hour, remove the solvent by rotary evaporation, and dry in vacuum for more than 12 hours (20-30°C) to obtain paclitaxel lipid complex I, sealed package , stored in the refrigerator.
[0075] (2) Preparation of Paclitaxel Lipid Complex II (Paclitaxel:Cholesterol (w / w)=1:2)
[0076] Take 0.5g of paclitaxel and 1.0g of cholesterol, add 200mL of acetone preheated to 40°C, compound at 40°C for 2 hours, remove the solvent by rotary evaporation, and dry in vacuum for more than 12 hours (20-30°C) to obtain paclitaxel lipid compound Material II, airtightly packaged, stored in the refrigerator.
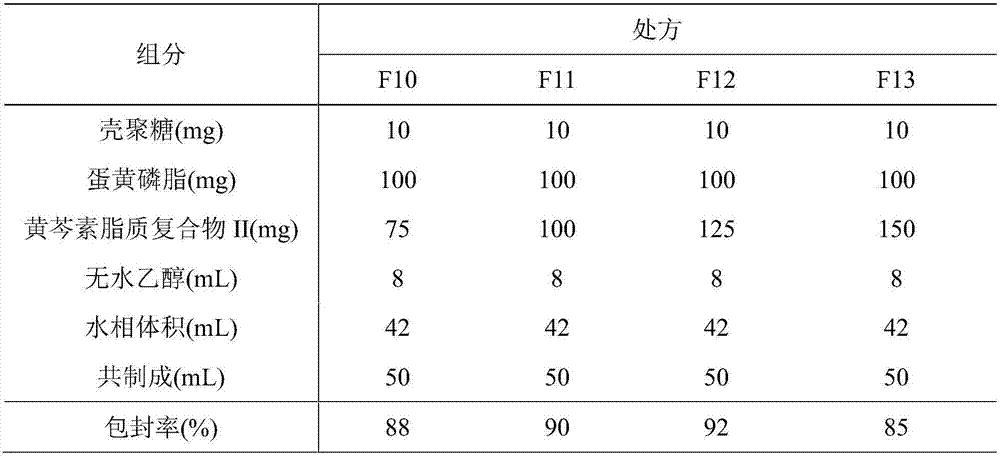
[0077] 2. Preparation of paclitaxel lipoplex II-phospholipid / chitosan drug delivery system
[0078] (1) low-viscosity chitosan is dissolved in ...
Embodiment 3
[0090] 1. Preparation of Chlorogenic Acid Lipid Complex
[0091] (1) Preparation of chlorogenic acid lipid complex I (chlorogenic acid: soybean lecithin (w / w)=1:2.5)
[0092] Take 0.5g of chlorogenic acid and 1.25g of soybean lecithin, add 50mL of absolute ethanol, compound at 40°C for 30 minutes, remove the solvent by rotary evaporation, and dry in vacuum for more than 12 hours (20-30°C) to obtain chlorogenic acid lipid compound Thing I, airtightly packaged, puts into the refrigerator for refrigerated preservation.
[0093] (2) Preparation of chlorogenic acid lipid complex II (chlorogenic acid:distearoylphosphatidylcholine (w / w)=1:2.2)
[0094] Take 1g of chlorogenic acid and 2.2g of distearoylphosphatidylcholine, add 50mL of absolute ethanol, compound at 40°C for 30 minutes, remove the solvent by rotary evaporation, and dry in vacuum for more than 12 hours (20-30°C), to obtain green Ortho-acid lipid complex II, airtightly packaged, stored in the refrigerator.
[0095] 2. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com