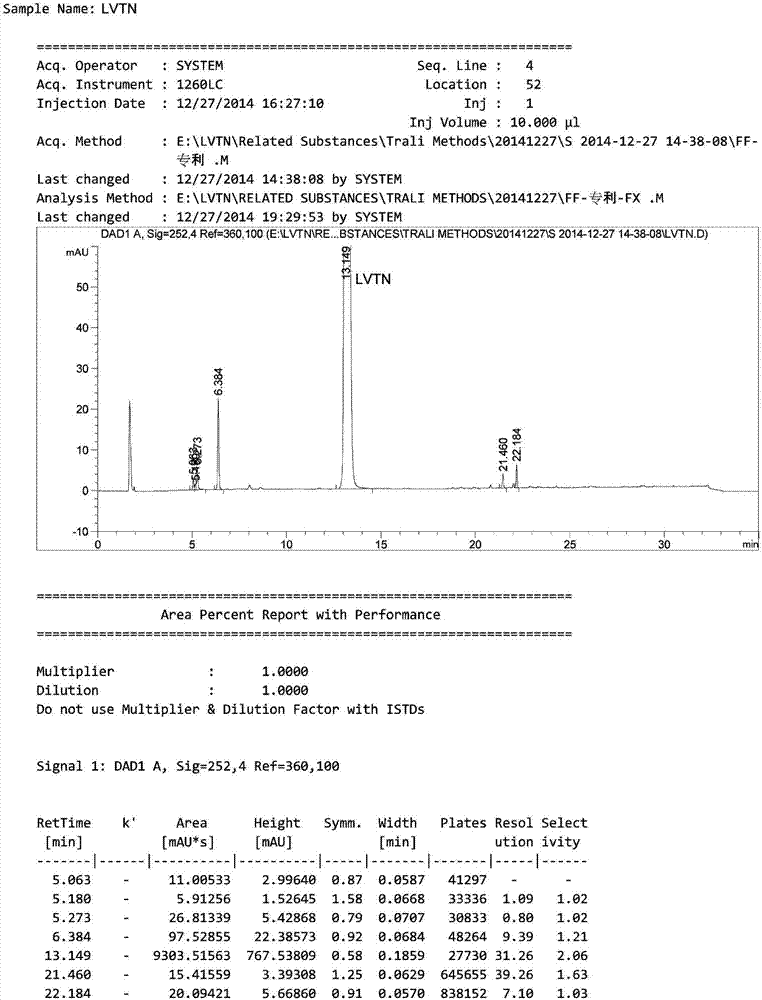
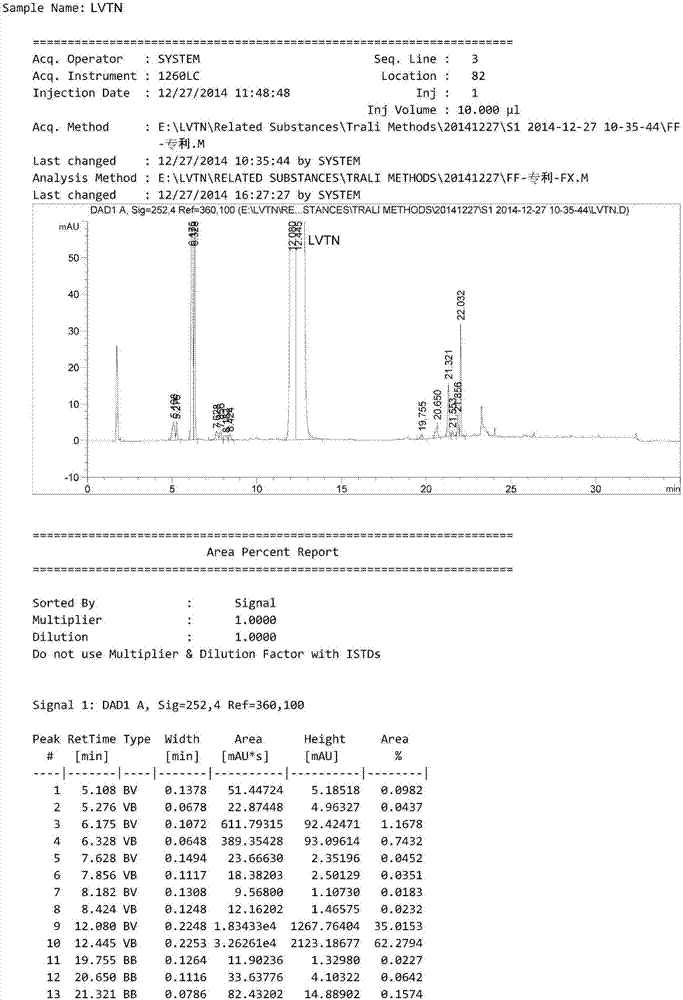
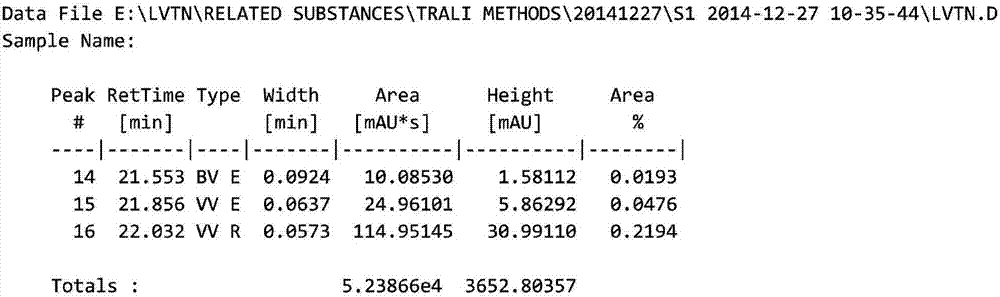
HPLC method for analyzing lenvatinib mesylate and preparation impurity thereof and application of impurity as reference standard
A lenvatinib and impurity technology, applied in the field of drug synthesis, can solve the problems that cannot truly reflect the quality of the drug, cannot accurately determine the true content of impurities, and has no qualitative analysis of impurities, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] Example 1: Synthesis of 4-(4-amino-3-chlorophenoxy)-7-methoxyquinoline-6-carboxylic acid amide (LVTN-1).
[0144] Under the protection of nitrogen, add 200mL dimethyl sulfoxide to a 500mL three-necked flask, start stirring, and add 20.00g 4-chloro-7-methoxyquinoline-6-carboxylic acid amide (SM1), 18.20g 4 - Amino-3-chlorophenol (SM2) and 14.22 g potassium tert-butoxide. After the addition, the temperature was raised to 65° C., and the mixture was kept stirring for 19 hours. The above reaction system was poured into pure water, a large amount of solids precipitated, filtered, the filter cake was rinsed with pure water, and air-dried at 60°C for 1 to 2 hours to obtain 25.07 g of brown solid 4-(4-amino- 3-Chlorophenoxy)-7-methoxyquinoline-6-carboxylic acid amide (LVTN-1), yield: 86.32%. Intermediate-1 1 H-NMR and high-resolution mass spectrometry see respectively image 3 and Figure 4 .
[0145] 1 H-NMR (400Mz, DMSO) δ: 8.685(s, 1H), 8.653(d, J=3.6Hz, 1H), 7.754-7....
Embodiment 2
[0146] Example 2: 4-(3-chloro-4-(N'-cyclopropylureido)phenoxy)-7-methoxyquinoline-6-carboxylic acid amide (LVTN-3) lenvatinib Synthesis
[0147] Add 250mL of N-methylpyrrolidone to a 500mL three-necked flask, start stirring, and then add 11.50g of pyridine and 25.00g of 4-(4-amino-3-chlorophenoxy)-7-methoxyquinoline-6- Carboxylic acid amide (LVTN-1). Under an ice-water bath, 4.94 g of phenyl chloroformate was added dropwise to the system. After the dropwise addition, the system was warmed up to room temperature. Add pure water dropwise to the reaction system, add dropwise for 1 to 2 hours, filter, rinse, and blow dry at 60°C for 2 hours to obtain 33.74g (4-((6-carboxycarboxamido-7 -Methoxyquinolin-4-yl)oxy)-2-chlorophenyl)phenylcarbamate (LVTN-2) brown powder. Yield: 81.26%.
[0148] Add 300mL N-methylpyrrolidone to a 1000mL three-necked flask, start stirring, and then add 30.00g (4-((6-carboxyformamido-7-methoxyquinolin-4-yl)oxy)-2- Chlorophenyl) phenyl carbamate (LVTN-2...
Embodiment 3
[0149] Example 3: Preparation of lenvatinib mesylate, 4-(3-chloro-4-(N'-cyclopropylureido)phenoxy)-7-methoxyquinoline-6-carboxylic acid Preparation of amide mesylate and preparation of lenvatinib mesylate capsules
[0150] Under the protection of nitrogen, add 100mL of acetic acid and 2.70g of methanesulfonic acid to a 250mL three-necked flask in sequence, and then add 10.00g of LVTN-3 to the system under temperature control at 40°C, stir for 1 to 2 hours, filter, collect the mother liquor, and The mother liquor was transferred to a 500mL three-neck flask, and under nitrogen protection, the temperature was controlled at 40°C, and 170mL of n-propanol was added dropwise to the system, and the dropping time was controlled for 1 to 2 hours. After filtering, the filter cake was rinsed with 70 mL of n-propanol to obtain the acetate compound of lenvatinib mesylate. Under nitrogen protection, add 100mL ethanol to a 250mL three-necked flask, start stirring, and add lenvatinib mesylate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com