A zinc complex based on 4-(2-methylimidazole) benzoic acid and its application
A technology of methylimidazole and zinc complexes, applied in zinc organic compounds, 2/12 group organic compounds without C-metal bonds, and other chemical processes, can solve the problems of storage research lag and achieve reproducibility Good, simple process, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Dissolve 4-(2-methylimidazole)benzoic acid (20.2mg, 0.1mmol), potassium hydroxide (5.6mg, 0.1mmol) and zinc nitrate (23.6mg, 0.1mmol) in water, and seal it in a reaction kettle, Heat to 110°C at a rate of 10°C per hour, maintain this temperature for 3 days, then cool down to room temperature naturally, wash with DMF, and filter through filter paper to obtain colorless blocky crystals. The obtained crystal products are placed in an oven at 80°C The temperature was kept at medium temperature for 3 hours, and the yield of the target product was about 65%. The main infrared absorption peaks are 3435(m), 3121(m), 1618(s), 1528(m), 1373(s), 1122(m), 1072(m), 845(m), 774(m) .
[0034] The zinc complex regulated by the mixed organic ligand prepared in Example 1 was further characterized, and the process is as follows:
[0035] (1) Determination of the crystal structure of the complex
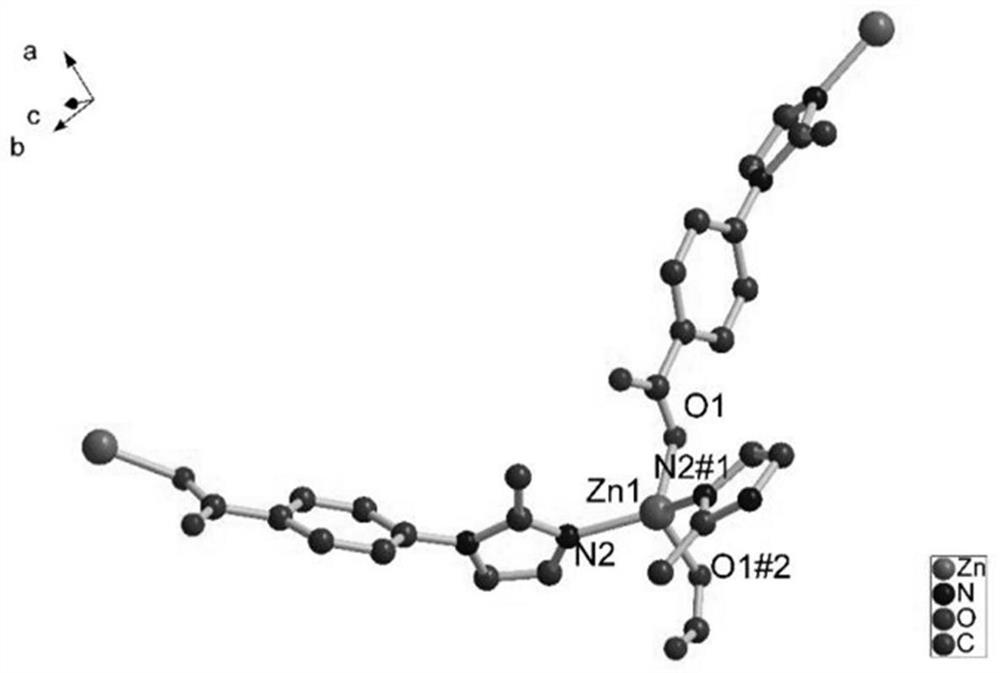
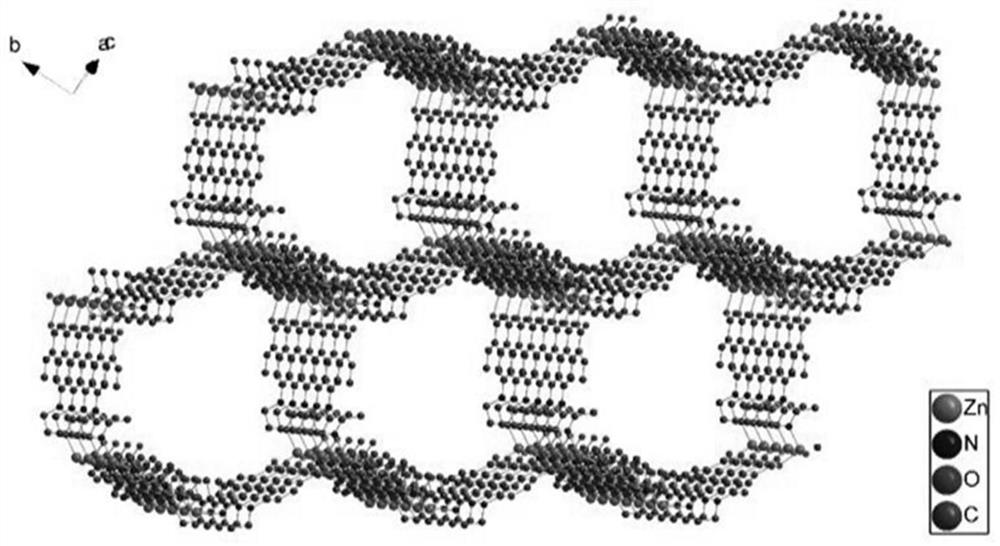
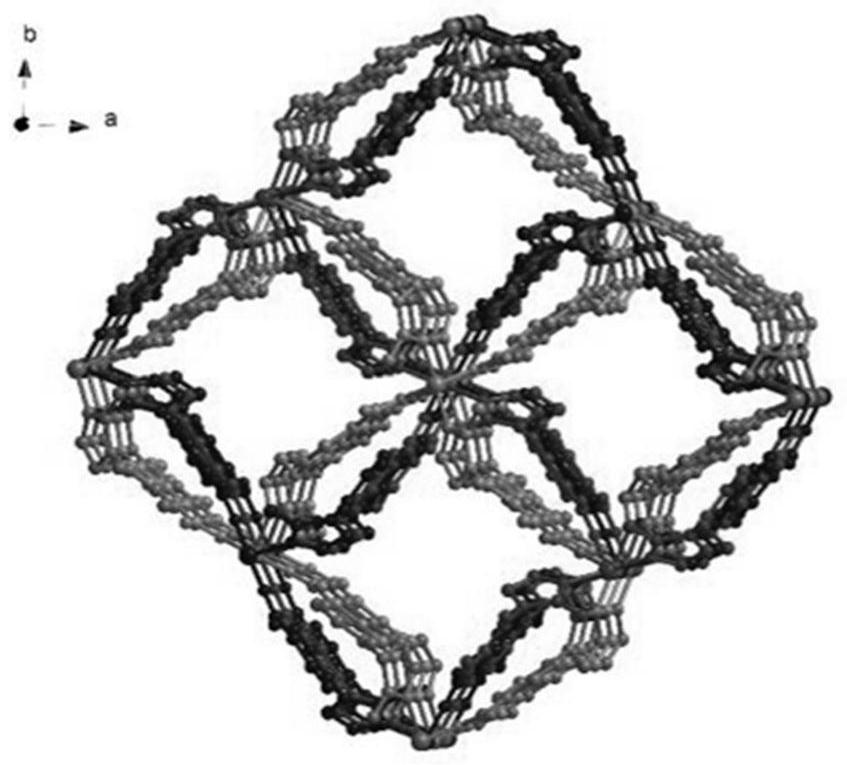
[0036] Under a microscope, a crystal with a suitable size of 0.32mm×0.30mm×0.28mm was sele...
Embodiment 2
[0054] 4-(2-Methylimidazole) benzoic acid (40.4mg, 0.2mmol), potassium hydroxide (11.2mg, 0.2mmol) and zinc nitrate (35.2mg, 0.15mmol) were dissolved in 10mL DMF solution, and the above mixture The solution was put into a reaction kettle lined with polytetrafluoroethylene, heated to 120°C at a rate of 6°C per hour, maintained at this temperature for 3 days, and then cooled to room temperature naturally to obtain colorless blocky crystals. The crystals were washed with DMF, and the target crystal product was obtained by filtration under reduced pressure, and the target product was placed in an oven at 80° C. for 3 hours at a constant temperature. Finally, the yield of the target product was about 75%.
Embodiment 3
[0056] 4-(2-Methylimidazole) benzoic acid (20.2mg, 0.1mmol), sodium hydroxide (11.2mg, 0.2mmol) and zinc nitrate (35.2mg, 0.15mmol) were dissolved in 10mL DMF solution, and the above mixture Put the solution into a reaction kettle lined with polytetrafluoroethylene, heat it to 130°C at a rate of 10°C per hour, maintain this temperature for 3 days, and then cool it down to room temperature naturally to obtain colorless blocky crystals. Rinse the crystals, obtain the target crystal product by filtration under reduced pressure, and place the target product in an oven at 80° C. for 3 hours at a constant temperature. Finally, the target product yield was about 67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com