Preparation method of bacterial polysaccharide and protein conjugate vaccine by using DSC as activating agent
A protein-binding vaccine, bacterial polysaccharide technology, applied in chemical instruments and methods, antibacterial drugs, bacterial antigen components, etc., can solve the problems of increased vaccine manufacturing cost, polysaccharide structure damage, long reaction period, etc., and achieve animal efficacy test results. Good, low free polysaccharide content, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
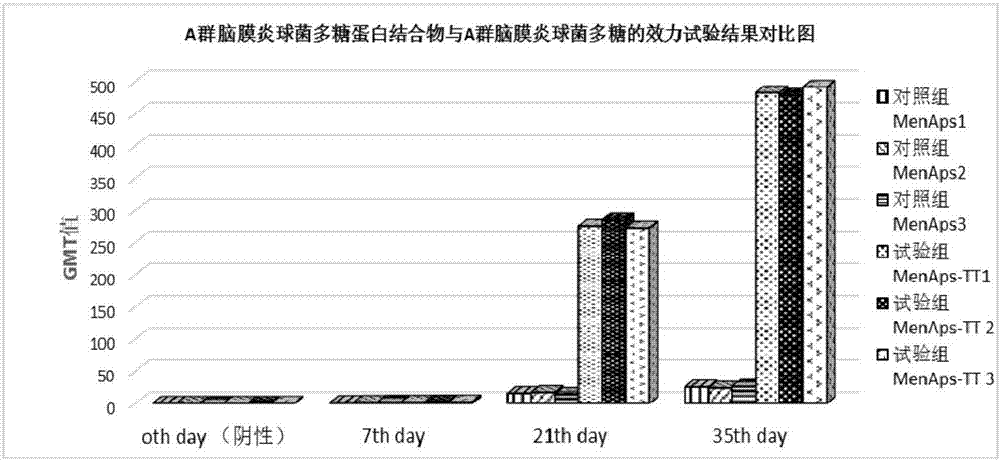
[0028] Example 1 Preparation of Group A Meningococcal Polysaccharide-protein Conjugate and Its Animal Effectiveness Test
[0029] Preparation of Polysaccharide Derivatives from Group A Meningococcus
[0030] Weigh 200 mg of the purified polysaccharide of meningococcus group A and dissolve it with 0.2 mol / L NaCl solution to 5 mg / ml; weigh 500 mg of DSC and dissolve it with acetonitrile to 100 mg / ml; adjust the polysaccharide of group A meningococcus The pH value of the solution is 8.0-9.5, add DSC powder, maintain the pH value at 8.0-9.5 and react for 10 minutes, add 800 mg of adipic hydrazide, maintain the pH value at 8.5-9.0 and react for 40 minutes; use a 30KD ultrafiltration membrane to concentrate by ultrafiltration, The polysaccharide derivative of group A meningococcus (MenAps-ADH) was obtained by lyophilization.
[0031] Preparation of Group A Meningococcal Polysaccharide-Protein Conjugate
[0032] Take group A meningococcal polysaccharide derivative 150mg, be dissolv...
Embodiment 2
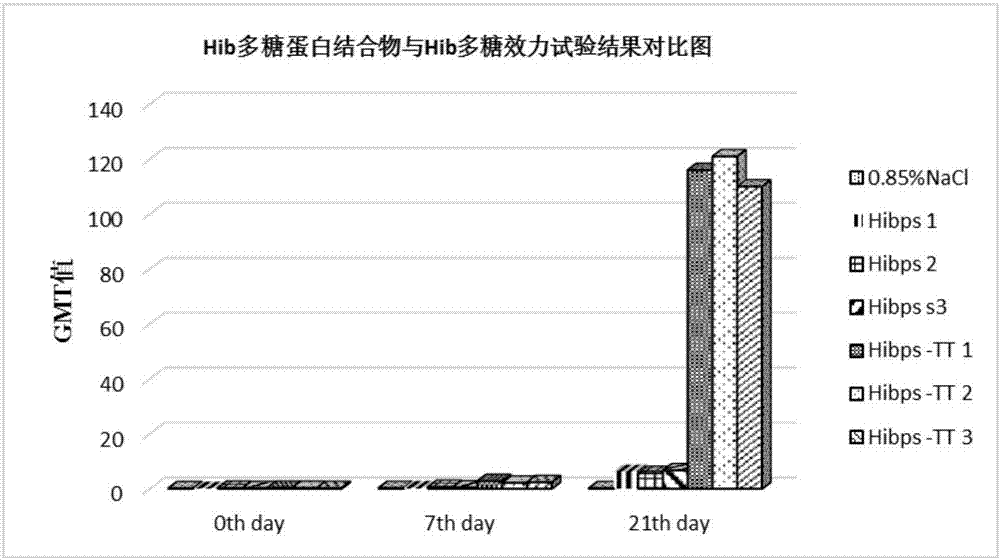
[0046] Example 2 Preparation of Haemophilus influenzae type b polysaccharide-protein conjugate and its animal effectiveness test
[0047] Preparation of polysaccharide derivatives from Haemophilus influenzae type b
[0048] Weigh 200 mg of purified Haemophilus influenzae type b polysaccharide, dissolve it with 0.2mol / L NaCl solution to 5 mg / ml; weigh 400 mg of DSC, dissolve it with acetonitrile to 100 mg / ml; adjust Haemophilus influenzae type b polysaccharide The pH value of the solution is 8.0-9.5, add DSC, maintain the pH value at 8.0-9.5 and react for 15 minutes, add 800 mg of adipic hydrazide, maintain the pH value at 8.5-9.0 and react for 20 minutes; concentrate with a 30KD ultrafiltration membrane, freeze Haemophilus influenzae type b polysaccharide polysaccharide derivative (Hibps-ADH) is obtained immediately after drying.
[0049] Preparation of Haemophilus influenzae type b polysaccharide-protein conjugate
[0050] Weigh 150 mg of Haemophilus influenzae type b polys...
Embodiment 3
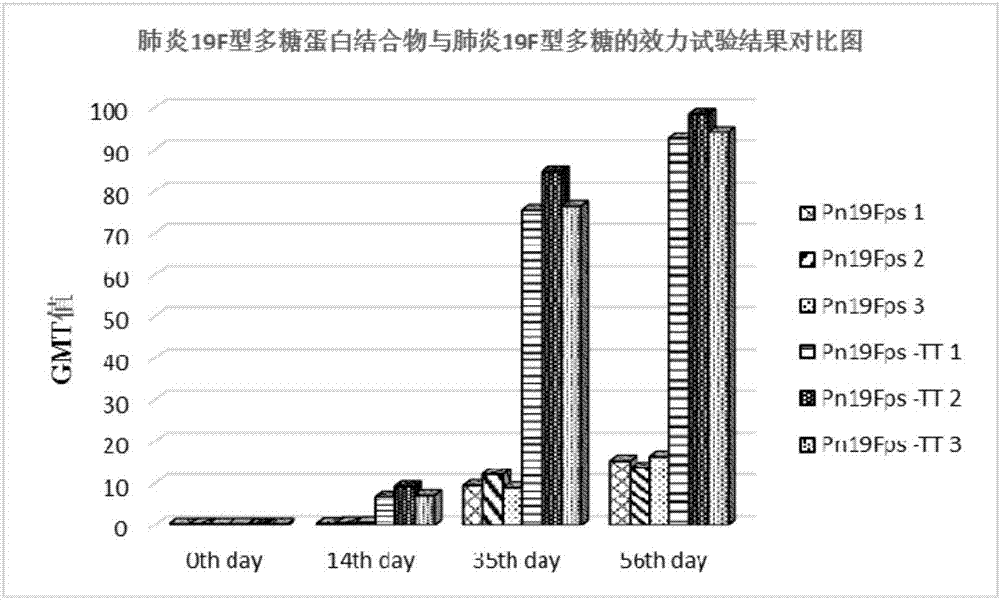
[0064] Example 3 Preparation of pneumococcal 19F polysaccharide-protein conjugate and its animal effectiveness test
[0065] Preparation of Pneumococcal 19F Polysaccharide Derivatives
[0066] Weigh 200 mg of purified pneumococcal polysaccharide 19F, dissolve it with 0.2 mol / L NaCl solution to 5 mg / ml; weigh 300 mg of DSC, dissolve it with acetonitrile to 100 mg / ml; adjust the pH of the pneumococcal 19F polysaccharide solution to 8.0-9.5, add DSC, maintain the pH value of 8.0-9.5 and react for 20 minutes, add 800 mg of adipic hydrazide, maintain the pH value of 8.5-9.0 and react for 20 minutes; use a 30KD ultrafiltration membrane to concentrate by ultrafiltration and lyophilize to obtain pneumonia Coccoid 19F-type polysaccharide derivative (Pn19Fps-ADH).
[0067] Preparation of pneumococcal 19F polysaccharide-protein conjugate
[0068] Take by weighing pneumococcal 19F type polysaccharide derivative 150mg, be dissolved in the NaCl of 15ml 0.2mol / L, add tetanus toxoid solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com