Oligosaccharide conjugate based on Streptococcus pneumoniae type 3 capsular polysaccharide, preparation method and applications thereof
A technology of Streptococcus pneumoniae and capsular polysaccharides, which is applied in the direction of medical preparations containing active ingredients, antibacterial drugs, drug combinations, etc., can solve problems such as poor biocompatibility, unpredictable impact of biological activity, and different effects. To achieve the effect of promoting antibody maturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
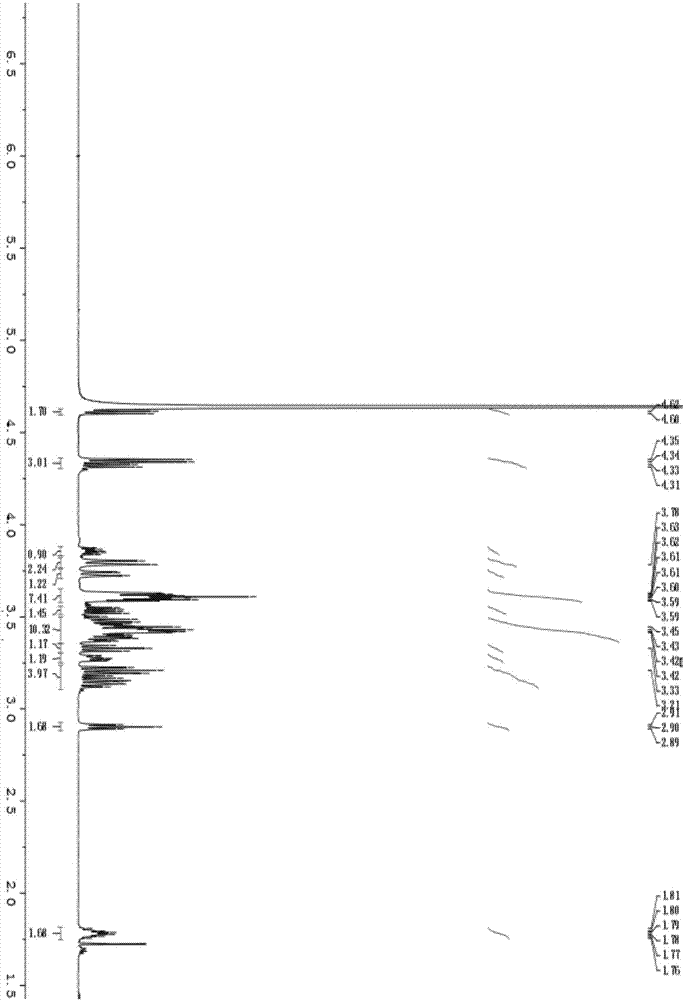
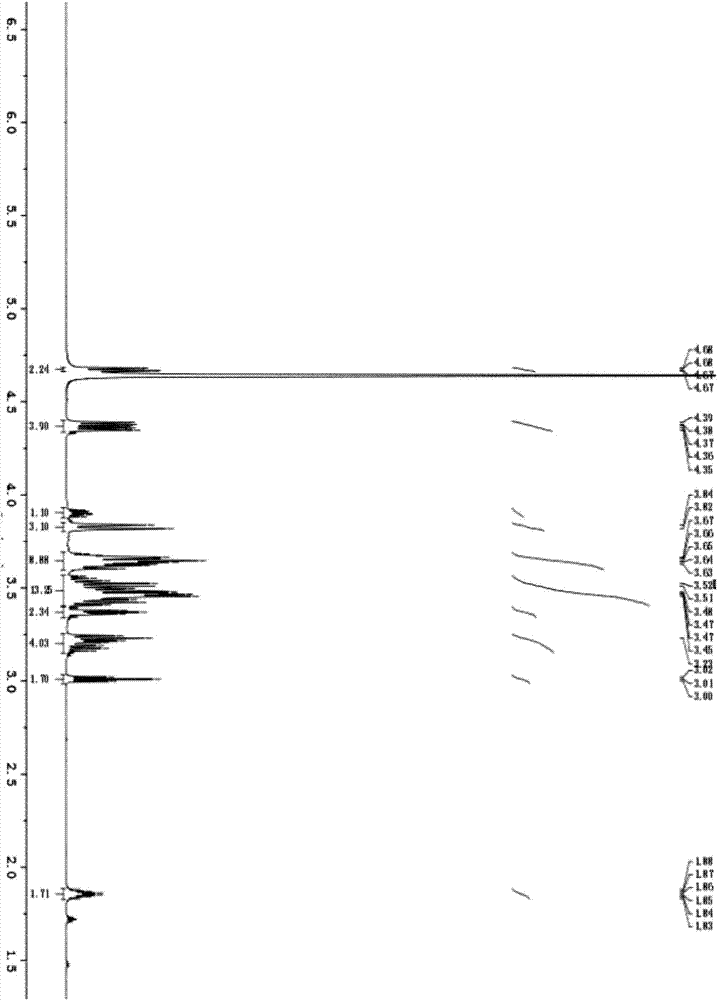
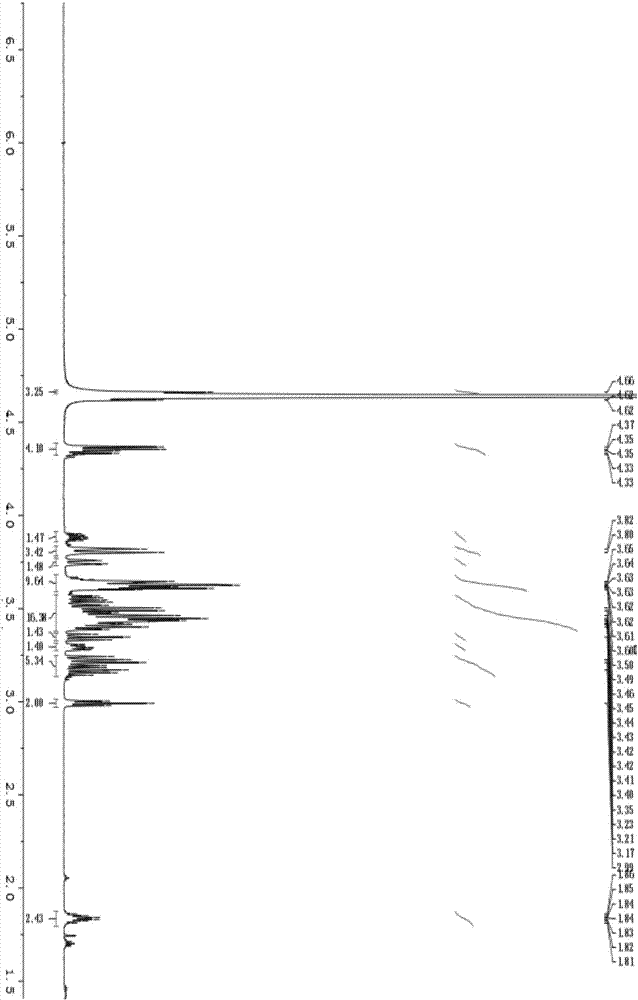
Image
Examples
Embodiment 1
[0042] Embodiment 1: general synthetic method
[0043] A: Benzoylation reaction
[0044] Get the raw material (1 mass part) and dissolve in pyridine, add benzoyl chloride solution (2.2 mass parts) under stirring condition, stir at room temperature for 2 hours, as the reaction proceeds, white solid is constantly separated out, concentrated, recrystallized, Filter to obtain a white solid product;
[0045] B: 4,6-position benzylidene transposition reaction
[0046] Take raw material (1 gram) and dissolve in dry 15 milliliters of dichloromethane, under the conditions of 0 ℃ and nitrogen protection, add triethylsilane (5 mass parts) and trifluoroacetic acid (5 mass parts), stir reaction 2 After 1 hour, dilute the reaction solution with 20 ml of dichloromethane, wash and extract with saturated aqueous sodium bicarbonate solution 3 times, take the lower organic phase, dry with anhydrous sodium sulfate, filter, concentrate, and separate with silica gel column chromatography. Deliqu...
Embodiment 2
[0056] Example 2: 3-aminopropyl β-D-glucosyl-(1→3)-β-D-glucosyl-(1→4)-β-D-glucosyl-(1→3)-β Synthesis of -D-glucosyl acid-(1→4)-β-D-glucose-TT conjugate (A17-TT)
[0057] (1) Synthesis of p-tolylthio 2,3,4,6-tetra-oxo-acetyl-1-thio-β-D-glucose (A1)
[0058]
[0059] Take β-D-full acetyl sugar (10.0g, 25.6mmol) and p-cresol (3.8g, 30.6mmol), after drying, dissolve in 50mL of dry dichloromethane, and add 10mL of Boron trifluoride ether solution, react at room temperature for 3 to 4 hours, TLC (PE:EA=1:2) to detect the reaction situation, until the raw materials no longer decrease, dilute the reaction solution with an appropriate amount of dichloromethane, and dilute the reaction solution with saturated NaHCO 3 After the aqueous solution was washed and extracted 3 times, the organic phase of the lower layer was removed and washed with anhydrous Na 2 SO 4 Dry, filter, concentrate, and separate by silica gel column chromatography to obtain compound A1 (10.0 g, yield 86%).
[...
Embodiment 3
[0112] Example 3: 3-Aminopropyl β-D-glucosyl-(1→4)-β-D-glucosyl-(1→3)-β-D-glucosyl-(1→4)- Synthesis of β-D-glucosyl-(1→3)-β-D-glucosyl-(1→4)-β-D-glucose-TT conjugate (B5-TT)
[0113] (1) 3-Azidopropyl 2,3-di-oxo-benzoyl-4,6-oxo-benzylidene-β-D-glucosyl-(1→4)-2,3-di- Synthesis of Oxy-benzoyl-6-oxo-benzyl-1-thio-β-D-glucose (B1)
[0114]
[0115] Using A4 (634.2 mg, 1.09 mmol) as the glycosyl donor and A5 (511.9 mg, 0.88 mmol) as the glycosyl acceptor, the target product B1 (676 mg, Yield 74%).
[0116] (2) 3-Azidopropyl 2,3-di-benzoyl-4,6-oxo-benzylidene-β-D-glucosyl-(1→4)-2,3-di-benzoyl Acyl-6-oxo-benzyl-β-D-glucosyl-(1→3)-2-oxo-benzoyl-4,6-oxo-benzylidene-β-D-glucosyl-(1→4 )-2,3-di-oxo-benzoyl-6-oxo-benzyl-β-D-glucosyl-(1→3)-2-oxo-benzoyl-4,6-oxo-benzyl Synthesis of -β-D-glucosyl-(1→4)-2,3-di-oxo-benzoyl-6-oxo-benzyl-1-thio-β-D-glucose (B2)
[0117]
[0118] Take B1 (355.6mg, 0.34mmol) as the glycosyl donor, A13 (491.9mg, 0.28mmol) as the glycosyl acceptor, carry ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com