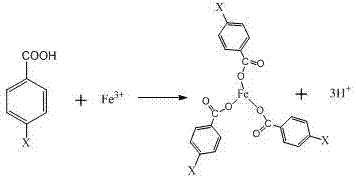
Method for separating chromium from iron in electroplating sludge leaching liquid through complexing precipitation
A technology for complexing sedimentation and electroplating sludge, applied in the fields of hydrometallurgy, materials, and chemistry, it can solve the problems of difficult separation of Cr and Fe, and achieve the effect of reducing the concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
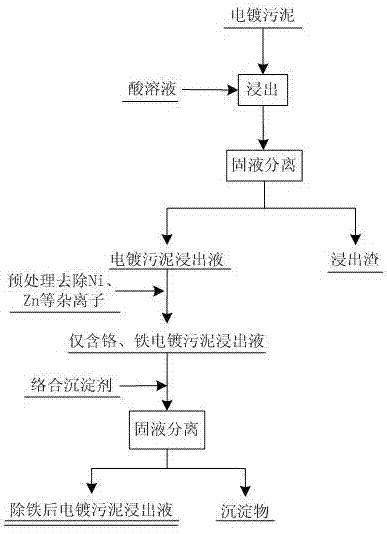
[0028] (1) The electroplating sludge leachate that has been pretreated to remove impurities such as Ni and Zn: iron content Fe 3+ 4g / L, chromium content Cr 3+ 3.8 g / L, solution pH=1.5.
[0029] (2) Pipette 100mL of leaching solution into a 300mL Erlenmeyer flask and put it into a constant temperature water bath with magnetic stirring. Weigh 2.62 g of benzoic acid and dissolve it in 10mL of ethanol, then slowly add it into the Erlenmeyer flask under constant temperature stirring at 30°C, and wait until the addition is complete. After stirring evenly, add 10% NaOH or other alkaline solutions to slowly adjust the pH of the rare earth solution to 2.5, continue to stir for 10 minutes, and then vacuum filter to obtain the filtrate. Through the above operations, Fe in the solution 3+ The content is 0.1g / L, the precipitation rate of iron is 95.43%, the loss of chromium is 3.2%, and the content of iron ions in the filtrate is significantly reduced.
Embodiment 2
[0031] (1) The electroplating sludge leachate that has been pretreated to remove impurities such as Ni and Zn: iron content Fe 3+ 5g / L, chromium content Cr 3+ 6g / L, solution pH=1.5.
[0032](2) Pipette 100mL of leaching solution into a 300mL conical flask and put it into a constant temperature water bath with magnetic stirring. Weigh 3.27 g of benzoic acid and dissolve it in 10mL of ethylene glycol, then slowly add it to the conical flask with constant temperature stirring at 30°C, and wait until Add 10% NaOH or other alkaline solutions to slowly adjust the pH of the rare earth solution to 2.5 after the feeding is completed and stir evenly. Continue to stir for 10 minutes and then vacuum filter to obtain the filtrate. Through the above operations, Fe in the solution 3+ The content is 0.26g / L, the precipitation rate of iron is 97.8%, the loss of chromium is not more than 2.9%, and the content of iron ions in the filtrate is significantly reduced.
Embodiment 3
[0034] (1) The electroplating sludge leachate that has been pretreated to remove impurities such as Ni and Zn: iron content Fe 3+ 5g / L, chromium content Cr 3+ 6g / L, solution pH=1.5.
[0035] (2) Pipette 100mL of leaching solution into a 300mL conical flask and put it into a constant temperature water bath with magnetic stirring. Weigh 4.476 g of p-nitrobenzoic acid and dissolve it in 15mL of methanol, then slowly add it into the conical flask at 30°C with constant temperature stirring. Add 10% NaOH or other alkaline solutions to slowly adjust the pH of the rare earth solution to 2.5 after the feeding is completed and stir evenly, continue to stir for 10 minutes and then vacuum filter to obtain the filtrate. Through the above operations, Fe in the solution 3+ The content is 0.2315g / L, the precipitation rate of iron is 96.37%, the loss of chromium is not more than 4.1%, and the content of iron ions in the filtrate is significantly reduced.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com