Enzymatic performance improved methyl parathion hydrolase mutant and application thereof
A technology of methyl parathion and mutants, applied in the field of genes encoding methyl parathion degrading enzyme mutants, mutants of organophosphate degrading enzymes, and methyl parathion hydrolase mutants, to improve enzyme The effects of chemical properties, widening of application fields and efficiency of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
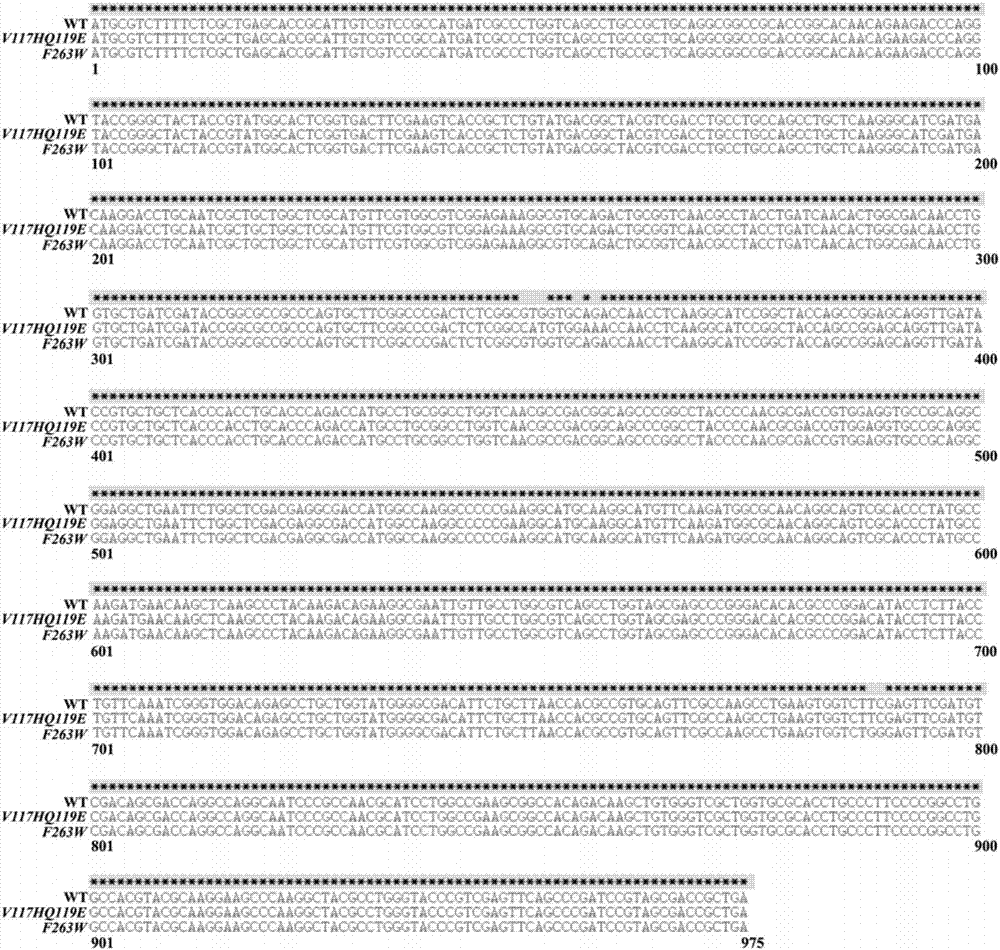
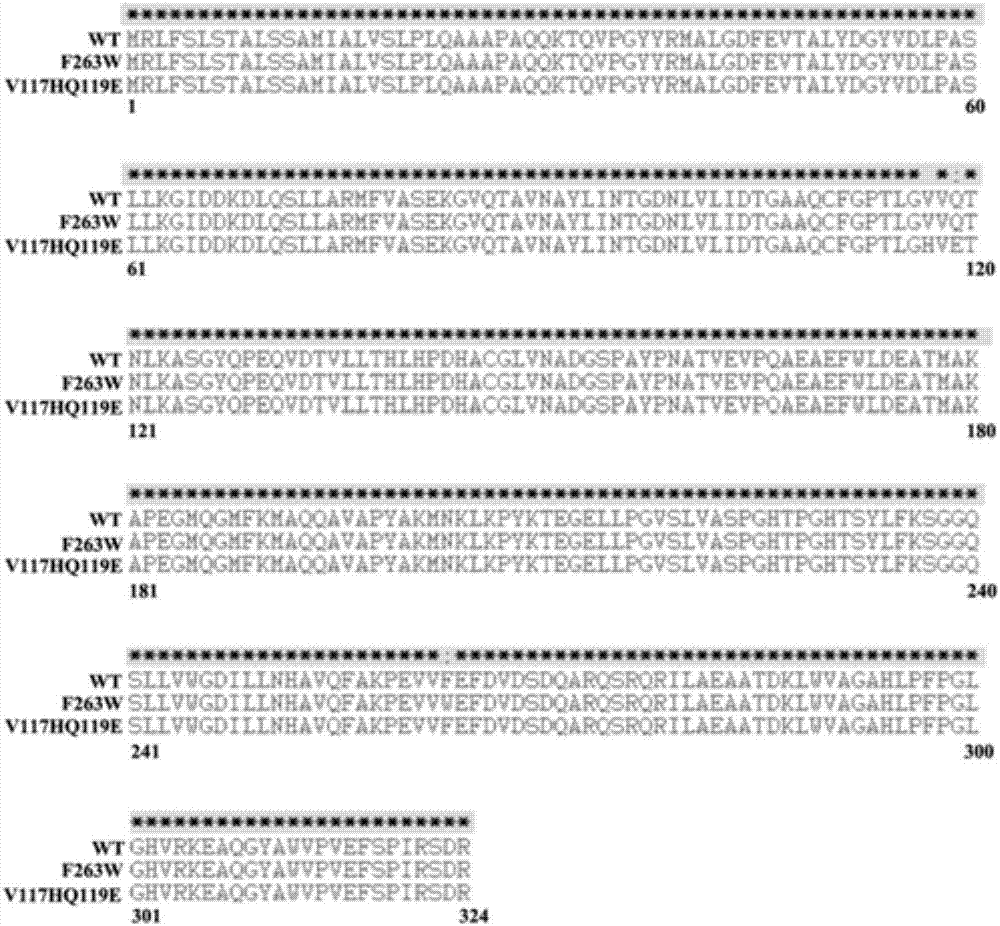
[0034] Example 1 Molecular transformation, mutant gene cloning and protein expression of methyl parathion degrading enzyme
[0035] 1. Test method
[0036] 1.1 Molecular simulation technology to determine the site to be mutated
[0037] 1.1.1 The I-tassel online program simulates the OPHC2 protein structure, and determines the candidate sites according to the structural characteristics of amino acid residues, hydrophobicity, isoelectric point, B-factor, and secondary structure;
[0038] 1.1.2 Use the Discovery studio software bulid mutant program to simulate the structure of the candidate point mutation site, and compare and analyze the tertiary structure force of the site before and after the mutation, and select the amino acid site with a more stable secondary structure after the mutation as a mutation target.
[0039] 2.1 Mutant gene cloning, expression and protein induction
[0040] 2.1.1 Design primers, add point mutation bases to the primer sequence, use the OPHC2 wil...
experiment example 2
[0061] Test of experimental example 2 mutant enzymatic properties
[0062] The relative enzyme activity is plotted, the pH curve, temperature curve and the measurement results of the influence of salt solution on the enzyme activity are shown in Figure 6-9 . The optimum action temperature of wild-type OPHC2 is 65°C, and the optimum action pH is 9.0. The pH curve and temperature response trend of the two mutant enzymes are consistent with wild-type OPHC2, but V117HQ119E shows good high temperature stability. When the temperature is higher than 65°C, the enzyme activity retention ability of the modified protein is significantly higher than that of the wild type. As the temperature increases, the advantage of heat resistance of the modified protein is more obvious. Wild type 13.5±0.7%, 80 ℃ higher than wild type 16±1.4% ( Figure 4 B).
[0063] At the same time, the mutant enzyme was subjected to time-gradient treatment at 65°C and 75°C, and after incubation at high temperatu...
experiment example 3
[0067] Experimental example 3 Stability detection experiment of mutant F263 in other surfactants
[0068] In view of the excellent stability of F263W in SDS, other surfactants were also selected to test the tolerance of F263W, and the treatment method was referred to SDS. The types of surfactants to choose from are as follows:
[0069] (1) Benzalkonium bromide, a cationic surfactant, is used in fields such as sterilization, disinfection, and algae killing;
[0070] (2) Apple amino acid foaming agent, compound amino acid surfactant, contains active substances extracted from apples, and is used in the fields of daily chemical and cleaning;
[0071] (3) Potassium cocoyl glycinate, an amino acid-type surfactant, used in the fields of daily chemicals and cleaning;
[0072] (4) Sodium lauroyl glutamate, an amino acid-type surfactant, used in the fields of daily chemicals and care;
[0073] (5) Firefighting foam Ⅰ: Composite foam fire extinguishing agent, the ingredients are fluor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com