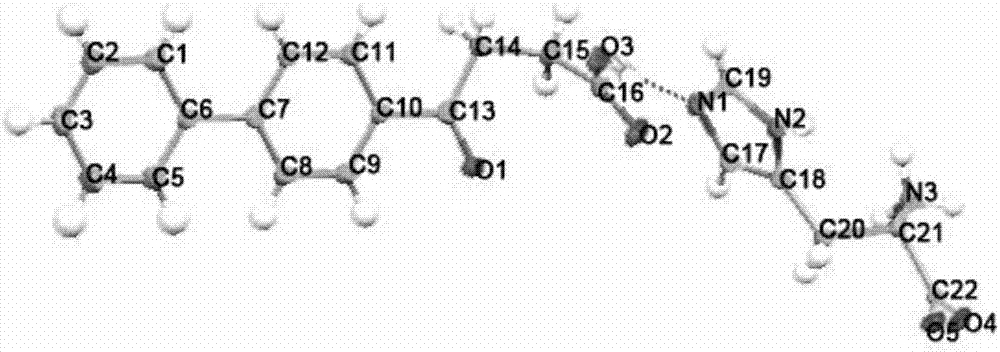
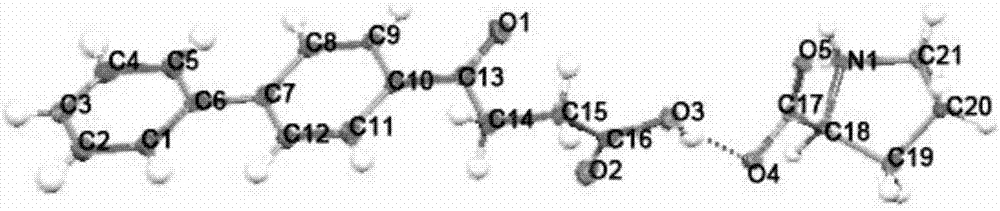
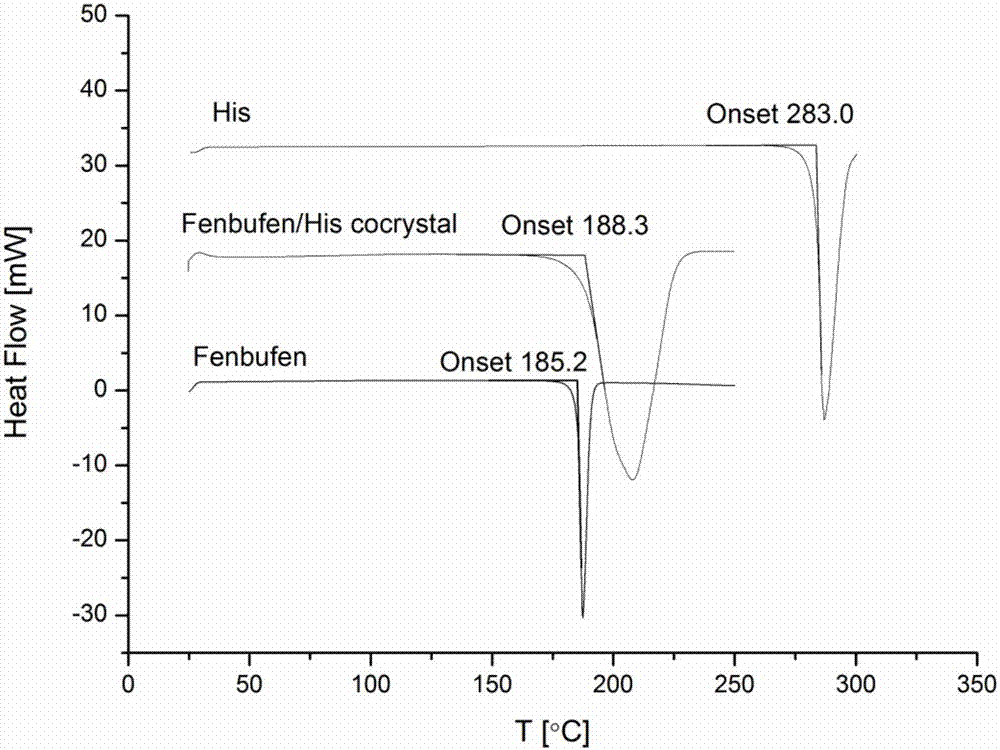
Fenbufen eutectic and preparation method and application thereof
A monoclinic and molecular technology, applied in organic chemistry methods, separation/purification of carboxylic acid compounds, organic chemistry, etc., can solve problems such as poor oral absorption and poor solubility, and improve solubility and bioavailability , low cost, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation method of the fenbufen co-crystal includes a solvent-assisted grinding method or a suspension method.
[0038] The solvent-assisted grinding method specifically includes the following steps: Feed the raw material fenbufen and the amino acid eutectic reagent at a molar ratio of 3:1 to 1:4, add the solvent dropwise, mix and grind for 10 to 300 minutes at a temperature of 10°C to 50°C, The fenbufen co-crystal was obtained.
[0039] When the amino acid co-crystal reagent is L-histidine, the solvent is selected from one of methanol, ethanol, water, acetonitrile, acetone, dimethyl sulfoxide, ethyl acetate and n-butanol.
[0040] When the amino acid co-crystal reagent is L-proline, the solvent is selected from ethanol, methanol, water, dimethylsulfoxide, dichloromethane, methanol-dimethylsulfoxide, water-dimethylsulfoxide-acetonitrile, and water- One of tetrahydrofuran-ethanol.
[0041] The suspension method specifically includes the following steps: take the...
example 1
[0046] Weigh 200.1mg fenbufen and 122.9mg L-histidine, add dropwise a small amount of dichloromethane, acetonitrile or n-butanol, grind for 20-300min, and dry to obtain fenbufen / L-histidine co-crystal .
example 2
[0048] Weigh 300.9 mg of fenbufen and 183.0 mg of L-histidine, add dropwise a small amount of dimethyl sulfoxide, methanol or water, grind for 20-300 minutes, and dry to obtain the fenbufen / L-histidine co-crystal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com