Biomass-based adsorption material for removing heavy metal ions in water as well as preparation method and application thereof
A technology of heavy metal ions and adsorption materials, applied in the directions of alkali metal compounds, alkali metal oxides/hydroxides, water pollutants, etc. Simple and high-efficiency reduction of heavy metal ion content and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
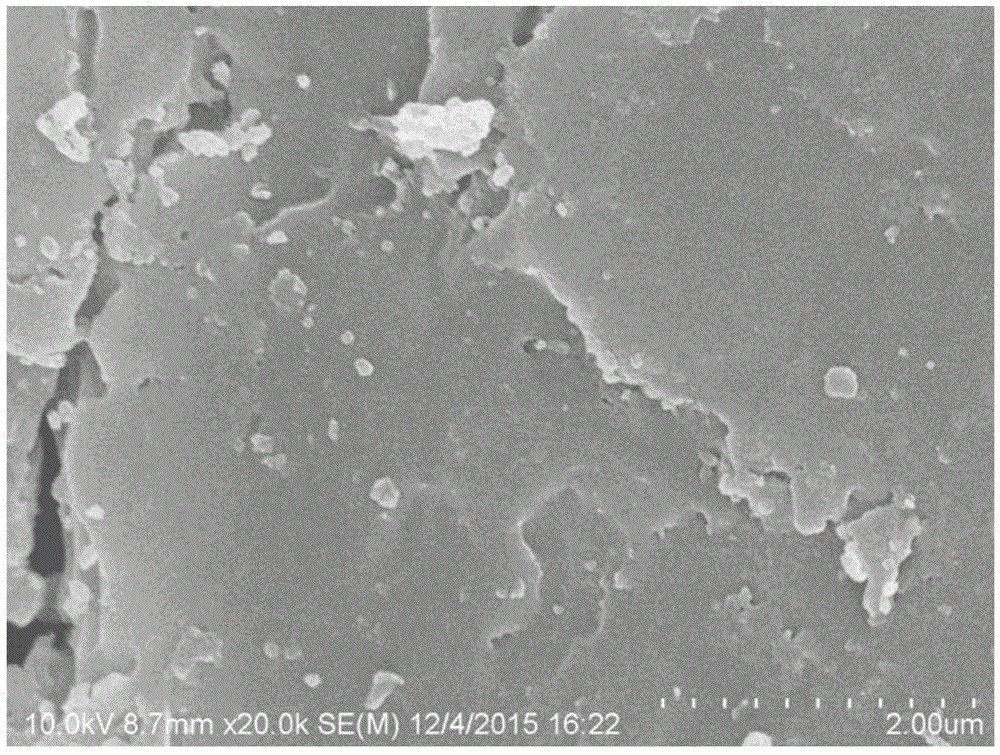
[0047] The specific surface area is 1.91m 2 The wheat straw / g is the biomass material powder obtained after the raw material is mechanically pulverized (the scanning electron microscope photo is shown in Fig. figure 1 shown), the preparation of biomass material / Fe 3 o 4 -Mg(OH) 2 (sample 1), and carry out the test of adsorption performance to sample 1.
[0048] In a 100mL flask, add 50mL distilled water, 3.2gMg(NO 3 ) 2 ·6H 2 O, 1.2g FeSO 4 ·7H 2 O, 2.2 g FeCl 3 ·6H 2 O, 0.8g of biomass material powder obtained by mechanical pulverization, fully stirred and dispersed, fed nitrogen for 5 minutes, began to slowly drip ammonia water, dripped ammonia water (25%) 10mL, and continued to feed nitrogen for 3 minutes. After the system was closed, it was placed in an oil bath at 70°C for 4 hours of reaction. After the reaction, the reaction product was repeatedly suction-filtered and washed until the pH value of the solution was neutral, and then the product was dried in an ov...
Embodiment 2
[0052] The specific surface area is 1.91m 2 / g wheat straw is the biomass material powder obtained by mechanical crushing of the raw material, and the biomass material / Fe 3 o 4 -Mg(OH) 2 (sample 2), and carry out the test of adsorption performance to sample 2.
[0053] In a 100mL flask, add 50mL distilled water, 3.2gMg(NO 3 ) 2 ·6H 2 O, 1.2g FeSO 4 ·7H 2 O, 2.2 g FeCl 3 ·6H 2 O, 1.0 g of biomass material powder obtained by mechanical pulverization, fully stirred and dispersed, fed nitrogen for 5 minutes, began to slowly drip ammonia water, dripped ammonia water (25%) 10mL, and continued to feed nitrogen for 3 minutes. After the system was closed, it was placed in an oil bath at 70°C for 6 hours. After the reaction, the reaction product was repeatedly suction-filtered and washed until the pH value of the solution was neutral, and then the product was dried in an oven at 70°C for 4 hours to obtain Biomass-based adsorption material sample 2 for removing heavy metal ions...
Embodiment 3
[0056] The specific surface area is 2.87m 2 / g wheat straw is the biomass material powder obtained by mechanical crushing of the raw material, and the biomass material / Fe 3 o 4 -Mg(OH) 2 (sample 3), and carry out the test of adsorption performance to sample 3.
[0057] In a 100mL flask, add 50mL distilled water, 3.2gMg(NO 3 ) 2 ·6H 2 O, 1.2g FeSO 4 ·7H 2 O, 2.2 g FeCl 3 ·6H 2 O, 1.5 g of biomass material powder obtained by mechanical pulverization, fully stirred and dispersed, fed nitrogen for 5 minutes, began to slowly drip ammonia water, dripped ammonia water (25%) 10 mL, and continued to feed nitrogen for 3 minutes. After the system was closed, it was placed in an oil bath at 50°C for 10 hours. After the reaction, the reaction product was repeatedly suction-filtered and washed until the pH value of the solution was neutral, and then the product was dried in an oven at 70°C for 4 hours to obtain Biomass-based adsorption material sample 3 for removing heavy metal io...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com