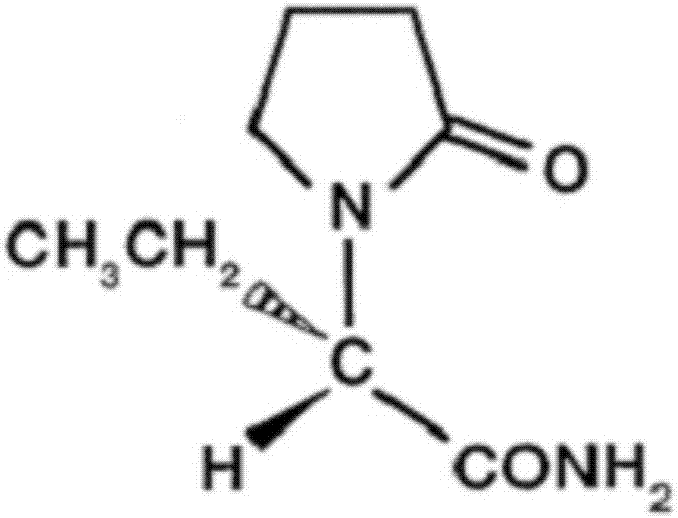
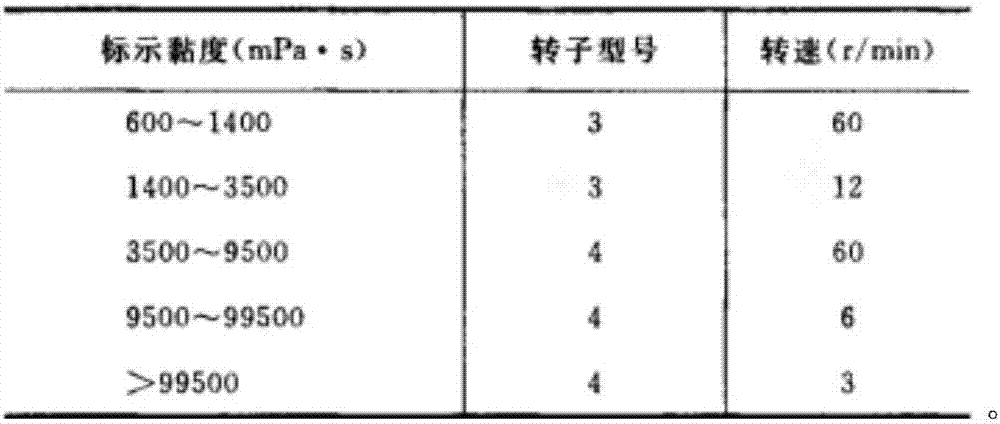
Levetiracetam sustained release tablet drug composition and quality control and preparation method thereof
A levetiracetam acid, slow-release tablet technology is applied in the field of levetiracetam sustained-release tablet pharmaceutical composition and its quality control and preparation method, which can solve the problem of large amount of levetiracetam and cannot be tableted. Satisfactory results have been achieved in the control of the growth rate of piracetam acid impurities, and the coating layer is prone to cracks and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Embodiment 1: the preparation of levetiracetam sustained-release tablet
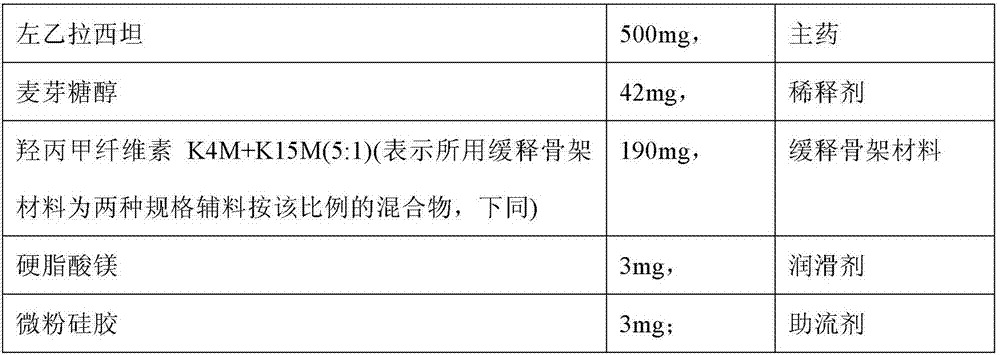
[0135] Tablet prescription:
[0136]
[0137] Preparation method: (1) making levetiracetam pre-crushed into a powder that can pass through a 100-mesh sieve, and making the rest of the materials pre-crushed into a powder that can pass through an 80-mesh sieve;
[0138] (2) Mix levetiracetam with the diluent and a part of the sustained-release framework material (the weight ratio of the part of the sustained-release framework material to the diluent is 0.75:1), spray in a fluidized state Enter 90% ethanol solution (its consumption is 4% of fluidized solid material weight) to make this mixed material bond into granule, make it seal and place 24 hours;
[0139] (3) Continue to fluidize to remove the ethanol solution (to the extent that the water content of the particles drops below 2%), then extrude the resulting material into a block through an extruder, and then crush the powder so that it can p...
Embodiment 2
[0144] Embodiment 2: the preparation of levetiracetam sustained-release tablet
[0145] Tablet prescription:
[0146] Levetiracetam
500mg,
50mg,
Hypromellose K4M
170mg,
4 mg,
Micropowder silica gel
2 mg;
[0147] Preparation method: (1) making levetiracetam pre-crushed into a powder that can pass through a 100-mesh sieve, and making the rest of the materials pre-crushed into a powder that can pass through an 80-mesh sieve;
[0148] (2) Mix levetiracetam with the diluent and a part of the sustained-release matrix material (the weight ratio of the part of the sustained-release matrix material to the diluent is 0.5:1), and spray in a fluidized state Enter 85% ethanol solution (its consumption is 6% of fluidized solid material weight) to make this mixed material bond into granule, make it seal and place 24 hours;
[0149] (3) Continue to fluidize to remove the ethanol solution (to the ext...
Embodiment 3
[0154] Embodiment 3: the preparation of levetiracetam sustained-release tablet
[0155] Tablet prescription:
[0156] Levetiracetam
500mg,
30mg,
Hypromellose K4M+K100M (5:1)
220mg,
2 mg,
Micropowder silica gel
4 mg;
[0157] Preparation method: (1) making levetiracetam pre-crushed into a powder that can pass through a 100-mesh sieve, and making the rest of the materials pre-crushed into a powder that can pass through an 80-mesh sieve;
[0158] (2) Mix levetiracetam with the diluent and a part of the sustained-release matrix material (the weight ratio of the part of the sustained-release matrix material to the diluent is 1:1), and spray it in a fluidized state. Enter 88% ethanol solution (its consumption is 3% of fluidized solid material weight) and make this mixed material bond into granule, make it seal and place 24 hours;
[0159] (3) Continue to fluidize to remove the ethanol solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com