Preparation method of prednisone acetate and intermediate thereof
A technology of prednisone acetate and intermediates, which is applied in the field of preparation of steroid drugs and their intermediates, can solve the problems of unsatisfactory introduction, achieve low cost, improve selectivity, and reduce the pressure of environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] A preparation method of prednisone acetate, using large-scale industrial production of 17α-hydroxyprogesterone as a raw material, first introduces the 11-position hydroxyl and the 1,2-position double bond simultaneously through a mixed microbial fermentation method, and first obtains 11α, 17α- Dihydroxypregna-1, 4-diene-3,20-dione intermediate; then undergo oxidation reaction to obtain 17α-hydroxypregna-1,4-diene-3,11,20-trione, and finally Prepare prednisone acetate through upper iodine displacement reaction, and concrete steps are:
[0044] (1) The specific steps for preparing 11α, 17α-dihydroxyprogesterone from 17α-hydroxyprogesterone through mixed microbial fermentation method are as follows:
[0045] 1) Mycelium culture
[0046] a) Mycelia culture of Rhizopus niger
[0047] Add culture medium into the shake flask, insert Rhizopus niger spores, place on a shaker at 26°C and 160 rpm, cultivate for 24-36 hours, collect mycelia by centrifugation, and expand the culture...
Embodiment 1
[0065] A kind of preparation method of prednisone acetate pharmaceutical intermediate, described prednisone acetate pharmaceutical intermediate is 11α, 17α-dihydroxypregna-1,4-diene-3,20-dione, its specific preparation The steps are:
[0066] 1) Mycelium culture
[0067] a) Mycelia culture of Rhizopus niger
[0068] Add culture medium into the shake flask, insert Rhizopus niger spores, place on a shaker at 26°C and 160 rpm, cultivate for 24-36 hours, collect mycelia by centrifugation, and expand the culture in a fermenter for later use;
[0069] b) Culture of Arthrobacter simplex
[0070] Add culture medium into the shake flask, insert Arthrobacter simplex, place in a shaker at 30°C and 120rpm, cultivate for 24-36 hours, collect the bacteria by centrifugation, and expand the culture in a fermenter for later use;
[0071] 2) Mixed culture to obtain the target product
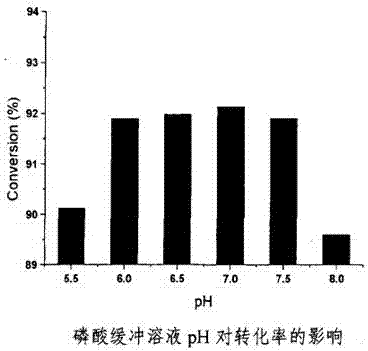
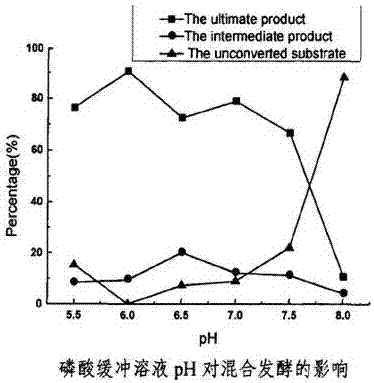
[0072] The resting cell transformation is carried out in an aqueous buffer solution with a pH of 5-7 and a...
Embodiment 2
[0074] A kind of preparation method of prednisone acetate pharmaceutical intermediate, described prednisone acetate pharmaceutical intermediate is 17α-hydroxy pregna-1,4-diene-3,11,20-trione, its specific preparation steps for:
[0075] 1) Preparation of DMP oxidant
[0076] In the preparation tank, add 7 parts by volume of sulfuric acid, add 1.5-2.4 parts by weight of o-iodobenzoic acid while stirring, keep stirring vigorously at 55 ° C, and add 1.4-2.2 parts by weight of potassium bromate, heat up to 65 ° C and stir for 3 -4 hours, cooled to 0°C, filtered and washed with ethanol, dried to obtain Compound A for future use;
[0077] Add 4 parts by volume of acetic anhydride, 0.1-0.5 parts by weight of p-toluenesulfonic acid of crystal water into the preparation tank, add compound A while stirring, heat up to 80°C and stir for 2 hours, cool to 0°C and filter, use CH 3 CH 2 -O-CH 2 CH 3 After washing, the oxidizing agent B can be obtained for subsequent use;
[0078] 2) Ox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com