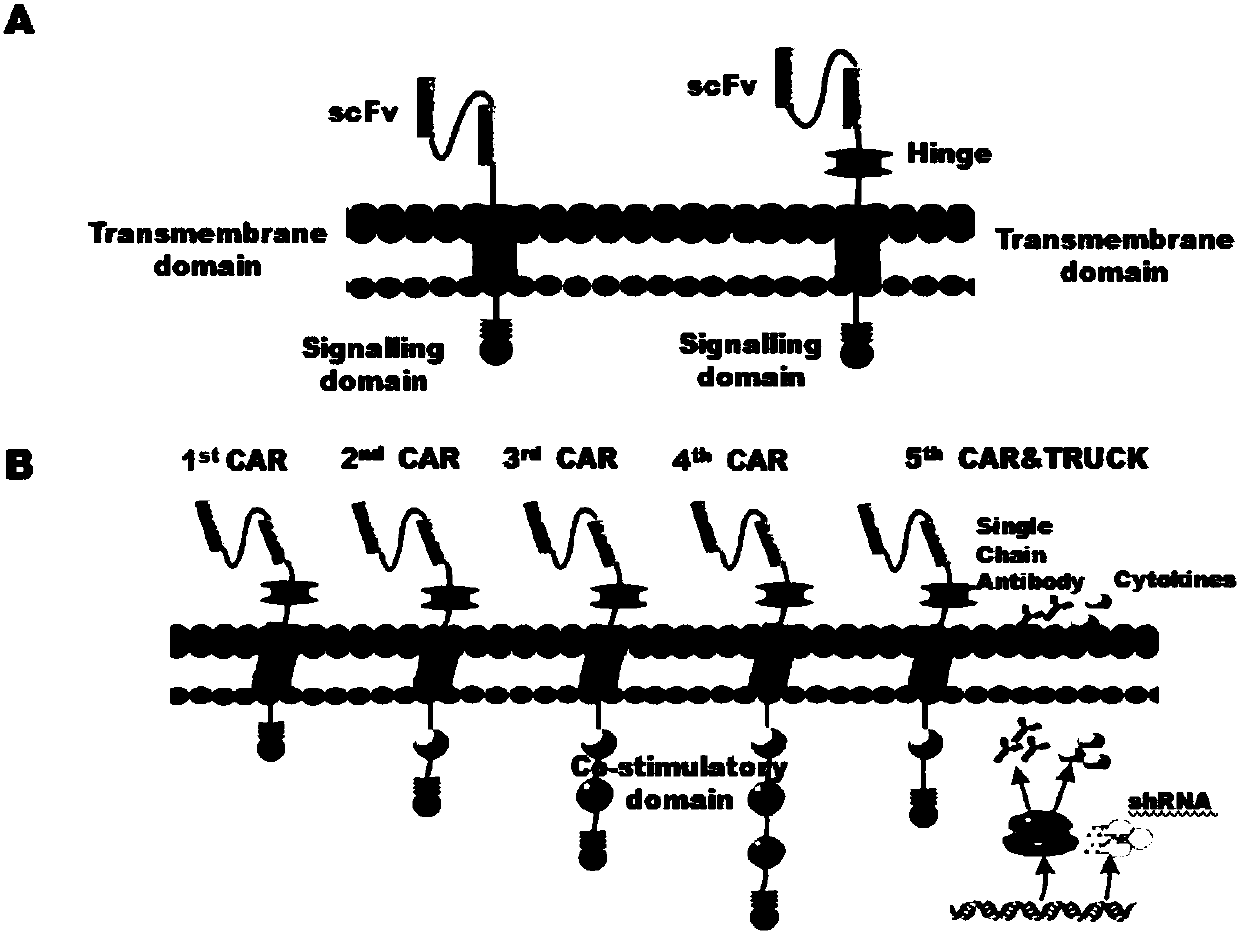
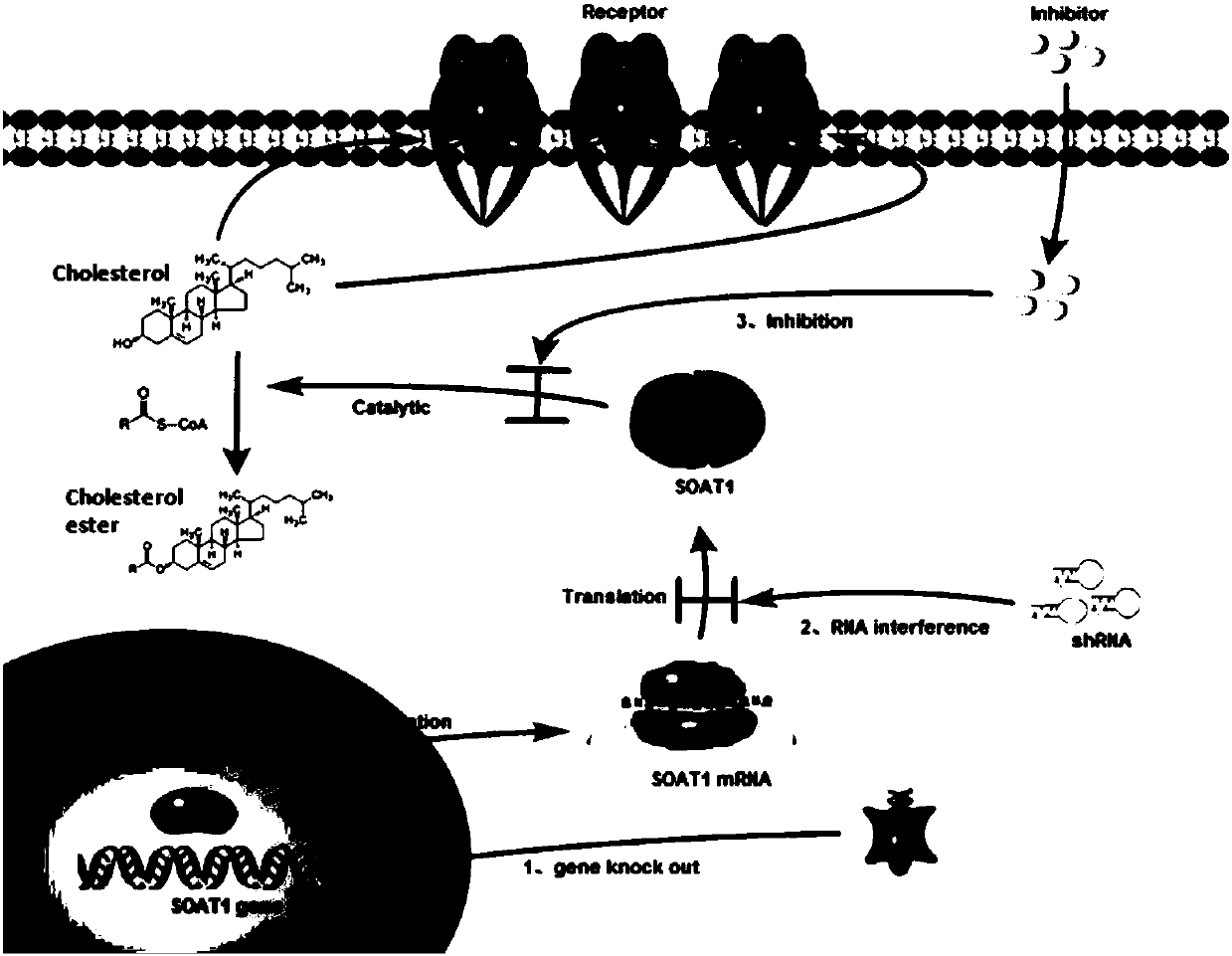
CAR-T cells inhibited by cholesterol translipase SOAT1, preparation method and application thereof
A SOAT1 and cholesterol technology, applied in the field of medical biology, can solve the problems of poor therapeutic effect and nutritional deficiency, and achieve the effect of increasing content, enhancing therapeutic activity and huge application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] Embodiment 1 hCAR19-KO SOAT1 - T cell construction.
[0155] see Figure 4 , the hCAR19-KO of the present invention SOAT1 -The construction method of T cells is as follows:
[0156]1. Construction, purification and detection methods of SOAT1 knockout recombinant lentiviral vectors lvCas9-SOAT1-1-lvCas9-SOAT1-7.
[0157] 1. Ligate the synthesized SOAT1-target1~SOAT1-target7 fragments into pLenti-Cas9-monoKO plasmids respectively to obtain SOAT1 knockout recombinant lentiviral plasmids pCas9-SOAT1-1~pCas9-SOAT1-7.
[0158] (1) The recombinant lentiviral plasmid pLenti-Cas9-monoKO was digested with BsmB I restriction endonuclease, the product was subjected to 1.5% agarose gel electrophoresis, and the fragment V1 of 11127bp was confirmed, and recovered by tapping the gel and placed in an Eppendorf tube, Reclaim the corresponding fragment (see Table 1) with the agarose gel recovery kit of MN company, and measure the purity and concentration of product;
[0159]
[01...
Embodiment 2
[0257] Example 2 hCAR19-shRNA SOAT1 - T cell construction.
[0258] see Figure 5 , the hCAR19-shRNA of the present invention SOAT1 -The construction method of T cells is as follows:
[0259] 1. Recombinant knockdown lentiviral vector lv-hCAR19-shRNA1 SOAT1 ~lv-hCAR19-shRNA13 SOAT1 Construction, purification and detection methods.
[0260] 1. The synthesized shRNA1 SOAT1 ~shRNA13 SOAT1 The fragments were co-ligated with the hU6 fragment into the p-hCAR19 recombinant lentiviral vector plasmid to obtain the SOAT1 recombinant knockdown lentiviral plasmid p-hCAR19-shRNA1 SOAT1 ~p-hCAR19-shRNA13 SOAT1 .
[0261] (1) The p-hCAR19 recombinant lentiviral vector plasmid was single-digested with Sal I restriction endonuclease, and the product was subjected to 1.5% agarose gel electrophoresis to confirm the 8519bp fragment V2, which was recovered by tapping the gel and placed in an Eppendorf tube. Recover the corresponding fragments (see Table 1 in Example 1) with the agarose g...
Embodiment 3
[0304] Example 3 hCAR19-T and hCAR19-Inhibitor SOAT1 - T cell construction.
[0305] see Figure 6 , the hCAR19-Inhibitor of the present invention SOAT1 -The construction method of T cells is as follows:
[0306] 1. Isolation of PBMCs.
[0307] (1) Take 50ml of fresh peripheral blood from a healthy donor;
[0308] (2) Spray the blood collection bag with alcohol twice and dry it.
[0309] (3) Use a 50ml syringe to suck out the blood cells in the bag and transfer them to a new 50ml tube.
[0310] (4) Centrifuge at 400g for 10 minutes at 20°C.
[0311] (5) Transfer the upper layer of plasma to a new 50ml centrifuge tube, inactivate the plasma at 56°C for 30 minutes, return to room temperature, centrifuge at 2000g for 30 minutes, and take the supernatant into a 50ml centrifuge tube for later use.
[0312] (6) Make up to 50ml with D-PBS(-), tighten the cap, and mix evenly by inversion.
[0313] (7) Take two new 50ml centrifuge tubes and add 15ml Ficoll lymphocyte separation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com