Bola type quercetin derivatives and preparation method and application thereof
A derivative, quercetin technology, applied in the field of chemical drug synthesis, can solve the problems of poor water solubility and fat solubility of quercetin, lack of tumor tissue targeting, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
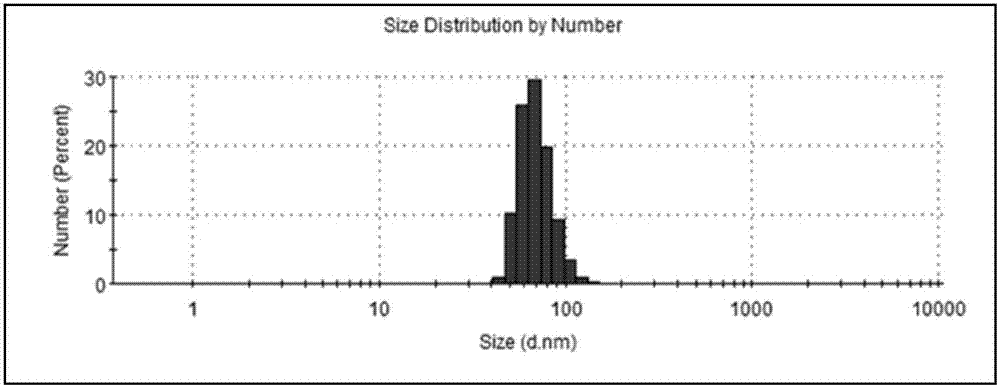
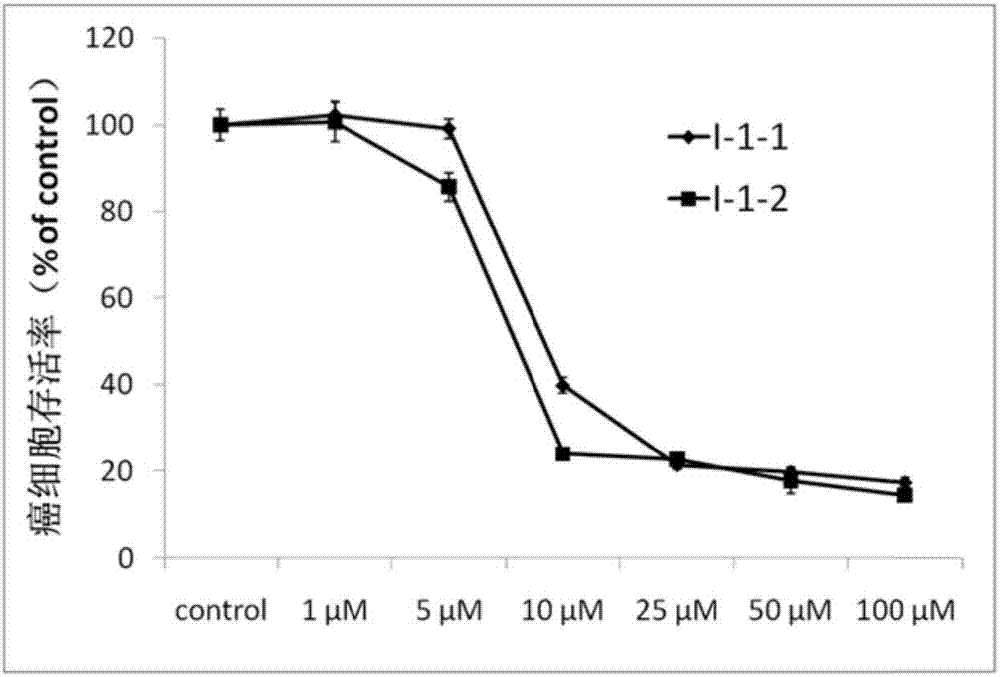
Image
Examples
Embodiment 1
[0056] Preparation of compound I-1-1
[0057] 1) Preparation of reaction intermediate VI-1
[0058]
[0059] Take 2.0g of rutin and place it in a round bottom flask, add 4.8g of cesium carbonate and 3.4g of benzyl bromide, then add 40ml of dimethylformamide and stir to dissolve. Heat to 60-90°C. After 48 hours, an aqueous acetic acid solution was added dropwise, and a solid precipitated out. After filtration, the filter cake was dissolved in ethanol solution of hydrochloric acid, heated to reflux for about 2 hours, and compound VI-1 was obtained after filtration.
[0060] VI-1: 1 H NMR (400MHz, CDCl 3 ):δ7.88(s,1H),7.76(d,1H,J=4Hz),7.32-7.61(m,20H),7.03(d,1H,J=4Hz),6.58(s,1H),6.48 (s,1H),5.24-5.26(m,6H),5.12(s,2H).
[0061] 2) Preparation of compound I-1-1
[0062]
[0063] Take compound VI-1 (according to 1.0eq), add 3.0eq cesium carbonate and 3.0eq 1,12-dibromododecane, use dimethylformamide as solvent, and stir for 8 hours. Compound I-1-1a was obtained by colu...
Embodiment 2
[0066] Preparation of compound I-1-2
[0067]
[0068] According to the method similar to Example 1, except that the 1,12-dibromododecane in the reaction was changed into 1,8-dibromooctane, the compound I-1-2 was obtained.
[0069] I-1-2: 1 H NMR (400MHz, DMSO-d 6 ): δ7.49(s,2H),7.40(s,2H),6.85(d,2H,J=4Hz),6.35(d,2H,J=4Hz),6.16(s,2H),3.89(t ,4H, J=8Hz), 1.60(t, 4H, J=8Hz), 1.19-1.28(m, 8H); 13 C NMR (100MHz, DMSO-d 6 ): δ178.42, 164.60, 61.71, 156.77, 156.37, 149.03, 145.58, 137.17, 121.37, 121.13, 115.97, 104.57, 98.95, 93.97, 72.42, 36.21, 31.25, 29.85, 25.8 [M+H] + .
Embodiment 3
[0071] Preparation of Compound 1-2
[0072]
[0073] Take compound VI-1 (according to 1.0eq), add 3.0eq cesium carbonate and 3.0eq 1,8-dibromooctane, use dimethylformamide as solvent, and stir for 8 hours. Compound VIII-1 was obtained by column chromatography. Compound VIII-1 was dissolved in methanol, stirred for 2 hours under the condition of palladium carbon / hydrogen, filtered, and the solvent was spin-dried to obtain compound IX-1. Dissolve compound IX-1 in acetonitrile, add potassium thioacetate, stir for 5 hours, and then separate by column chromatography to obtain compound X-1. Dissolve compound X-1 in methanol, add potassium phosphate, stir in air for 5 hours, then add 1 mol / L hydrochloric acid dropwise, then filter and separate by column chromatography to obtain compound I-2.
[0074] I-2: 1 H NMR (400MHz, DMSO-d 6 ): δ7.52(s,2H),7.43(d,2H,J=4Hz),6.90(d,2H,J=4Hz),6.44(s,2H),6.20(s,1H),3.90(s ,4H),2.66(d,4H,J=4Hz),1.60(s,8H),1.23-1.30(m,16H); 13 C-NMR (100MHz,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com