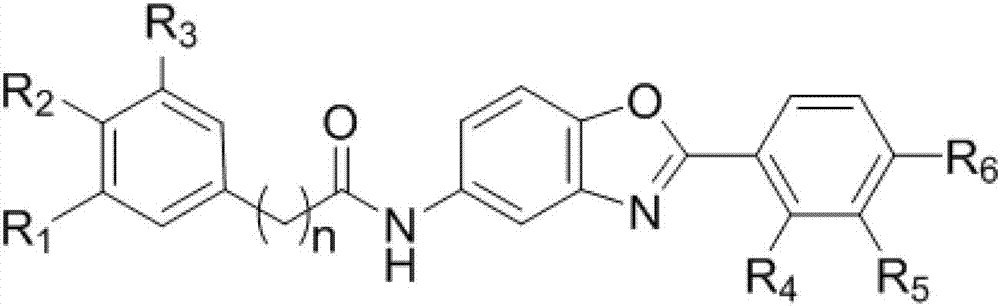
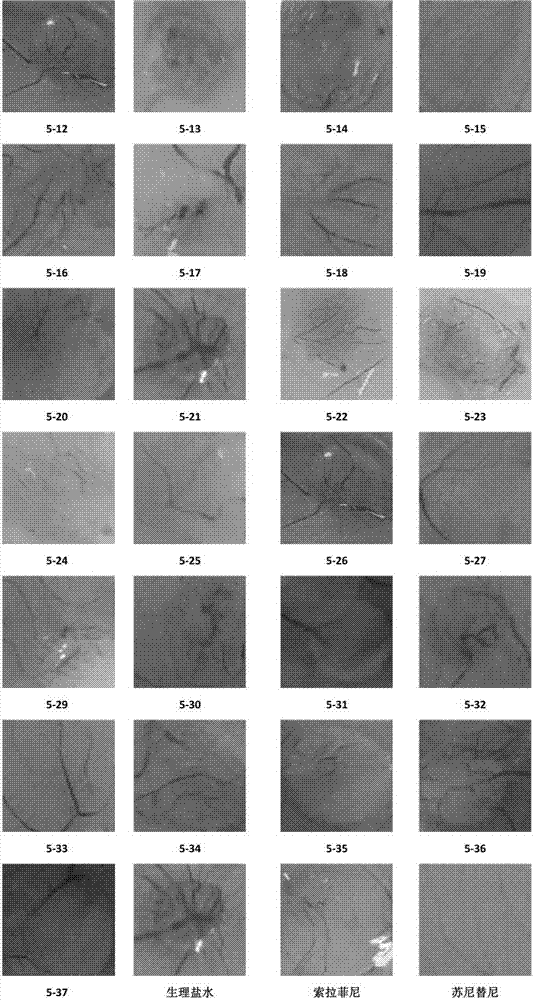
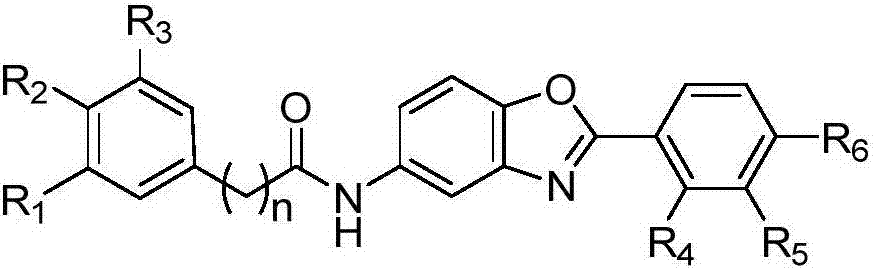
Compound capable of inhibiting conduction of vascular endothelial growth factor receptor signal
A growth factor receptor and signal transduction technology, applied in the field of medicine, can solve the problems of tumor cells lack of nutrition, stop growing, slow blood flow, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of compound 5-3 in the above Table 2, namely the preparation of 3-bromo-4-chloro-N-(2-phenyl-5-benzoxazolyl)benzamide.
[0047] The 3-bromo-4-chloro-N-(2-phenyl-5-benzoxazolyl)benzamide has the following chemical structural formula:
[0048]
[0049] The preparation method of described compound 5-3 is as follows:
[0050] Select 1mmol of substituted benzoic acid or substituted phenylacetic acid 4, add it to a 20mL round bottom flask, take 20mL of DMF to dissolve, then add 1.5mmol of HOBT, 1.5mmol of EDCI, 2mmol of TEA triethylamine in turn, and then use a normal temperature magnetic stirrer Stir for about 10 minutes, then add substituted 2-phenylbenzoxazole 3 dissolved in DMF, stir at room temperature, react for 2-4 hours, TLC detects that the reaction of raw materials is complete, stop stirring, add 15 mL of ethyl acetate for extraction, and then use 50 mL of dilute hydrochloric acid After washing twice, the organic phase was dried with anhyd...
Embodiment 2
[0052] Example 2: Preparation of Compound 5-1 in the above Table 2, namely the preparation of 4-bromo-3-fluoro-N-2-(2-phenylbenzoxazol-5-yl)benzamide. The preparation method is as in Example 1, and has the following chemical structural formula:
[0053]
[0054] NMR spectrum data: yellow solid; Mp 227.9–229.9°C; IR(KBr): 3334,3064,1650,1544,1483,1279,1046,870,764,690; 1 HNMR (500MHz, DMSO-d 6 ):δ=7.58-7.61 (d, J=7.20Hz, 3H, ArH), 7.73-7.96(m, 5H, ArH), 8.16-8.18(d, J=7.15Hz, 2H, ArH), 8.27(s ,1H,ArH),10.53(s,1H,ArH); 13 CNMR (125MHz, DMSO-d 6 ):δ=110.91, 111.80, 116.08, 116.27, 119.17, 125.63, 126.74, 127.58, 127.58, 129.59, 129.59, 132.24, 133.99, 136.26, 136.64, + ): m / z calcd for C 20 h 12 BrFN 2 o 2 [M+Na] + ,432.9952; found, 432.9958.
Embodiment 3
[0055] Example 3: Preparation of Compound 5-2 in the above Table 2, namely the preparation of 4-chloro-3-fluoro-N-2-(2-phenylbenzoxazol-5-yl)benzamide. The preparation method is as in Example 1, and has the following chemical structural formula:
[0056]
[0057] NMR spectrum data: yellow solid; Mp 216.9–218.5°C; IR(KBr): 3346,3064,1654,1548,1479,1426,1058,812,694,637; 1 H NMR (500MHz, DMSO-d 6 ): δ=7.60-7.62 (d, J=6.95Hz, 3H, ArH), 7.75-7.89 (m, 4H, ArH), 7.99-8.02 (d, J=10.00Hz, 1H, ArH), 8.17-8.20 (d, J=7.20Hz, 2H, ArH), 8.27(m, 1H, ArH); 13 C NMR (125MHz, DMSO-d 6 ): δ=110.97, 111.86, 116.35, 116.53, 119.25, 125.38, 126.74, 127.61, 127.61, 129.63, 129.63, 131.13, 132.29, 135.99, 136.26, 141.97, 1463.367, TO + ):m / z calcd for C 20 h 12 ClFN 2 o 2 [M+Na] + ,389.0466; found, 389.0464.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com