Gemfibrozil co-crystal and preparation method thereof
A technology of gemfibrozil and hexamethylenetetramine, applied in the field of drug development, can solve the problems of complex preparation process, poor stability, low drug loading and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~12
[0035] Examples 1-12 Preparation experiment and solubility test of gemfibrozil-isonicotine co-crystal
[0036] Take gemfibrozil and the corresponding isonicotine synthon in a molar ratio of 1:0.5 to 1:2 and place them in a ball mill jar, add 0.1 to 0.3ml of different auxiliary solvents and a stainless steel grinding ball with a diameter of 12mm, 1300 to 1800r The ball milled for 20-30 minutes under the vibration condition of / min. After the ball milling, the sample was dried naturally to obtain the gemfibrozil-isonicotine eutectic, and then the dissolution test was done in the in vitro buffer solution.
[0037] Table 1. Experimental parameters of gemfibrozil-isonicotine cocrystal, wherein the solubility of the original drug gemfibrozil is 0.22mmol / L.
[0038]
Embodiment 13~24
[0039] Example 13-24 Preparation of Gemfibrozil Hexamethylenetetramine Cocrystal
[0040] Take gemfibrozil and the corresponding hexamethylenetetramine synthon at a molar ratio of 1:0.5 to 1:2 and place them in a ball mill jar, add 0.1 to 0.3ml of different auxiliary solvents and a stainless steel grinding ball with a diameter of 12mm, Under the vibration condition of 1300~1800r / min, ball mill for 20~30min. After the ball milling, the sample was dried naturally to obtain Gemfibrozil hexamethylenetetramine eutectic, and then the dissolution test was done in the in vitro buffer solution.
[0041] Table 2. Experimental parameters of gemfibrozil hexamethylenetetramine cocrystal, wherein the solubility of the original drug gemfibrozil is 0.22mmol / L.
[0042]
[0043]
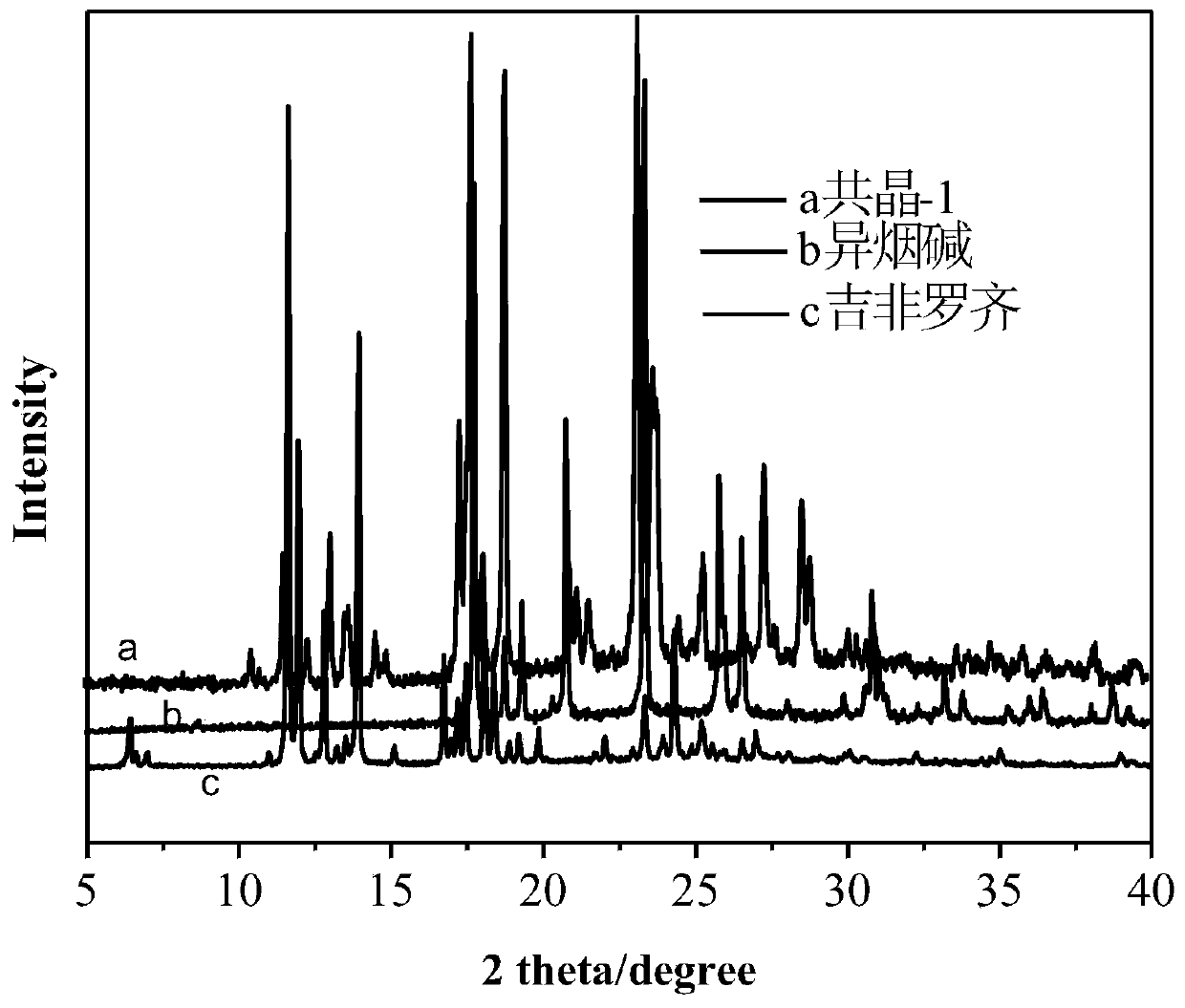
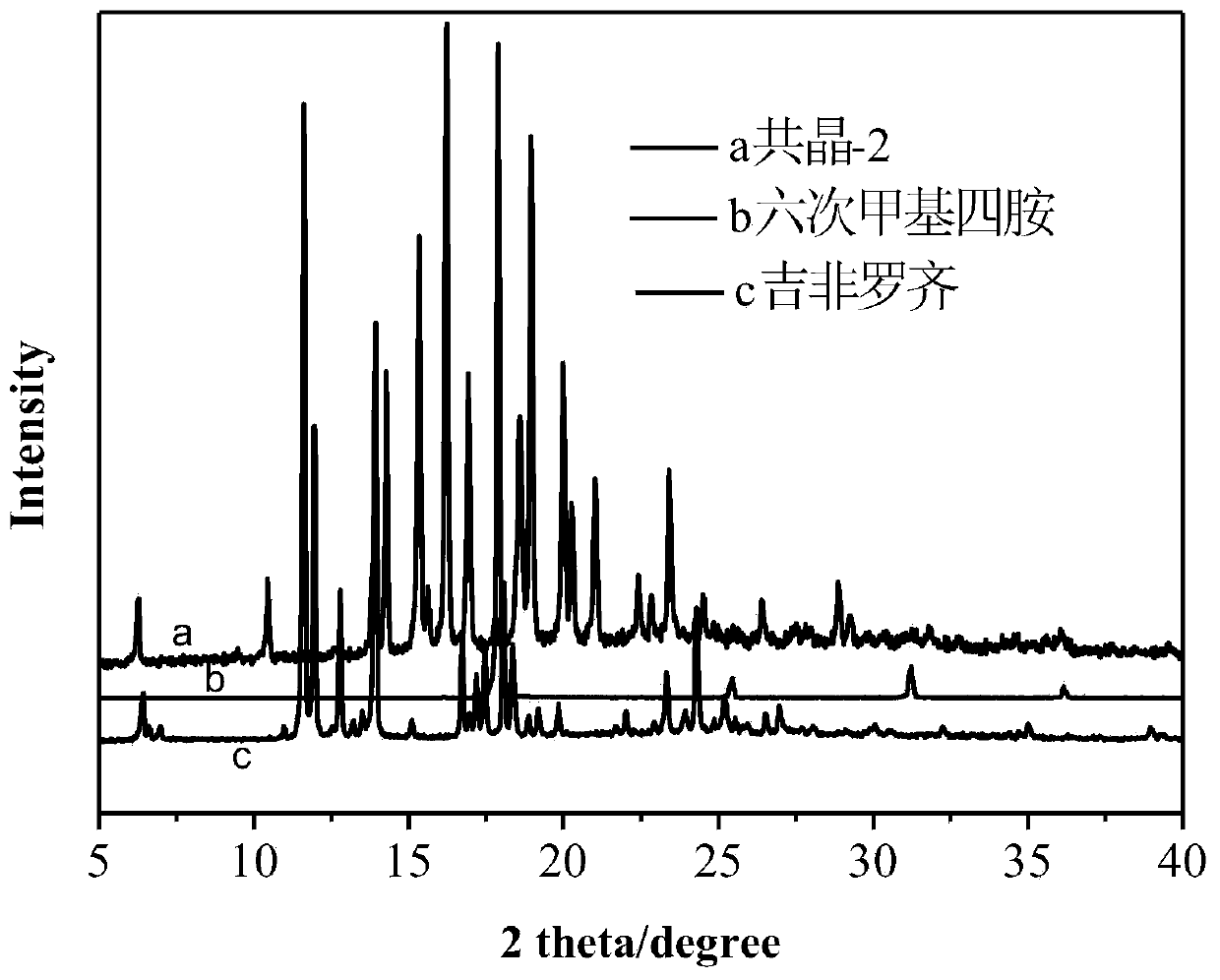
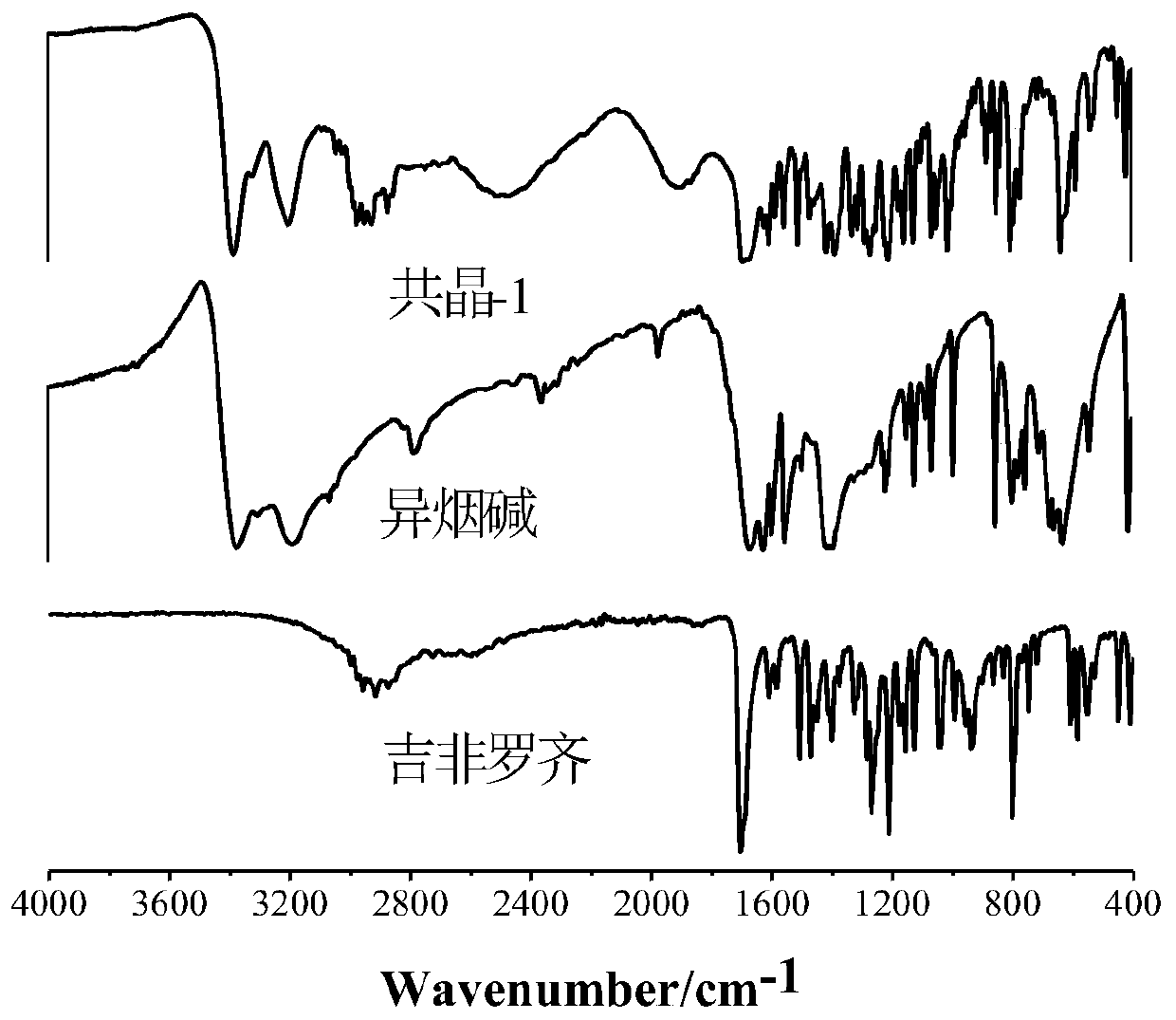
[0044] Gemfibrozil isonicotine and gemfibrozil hexamethylenetetramine cocrystals were analyzed by X-ray powder diffraction (PXRD), infrared (IR), thermogravimetric (DSC) 1 H NMR) for characterization, the stru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com