Treatment of ascites
A technique for ascites and patients, which is applied in medical preparations containing active ingredients, peptide/protein ingredients, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 : Treatment of ascites with continuous infusion pump terlipressin
[0024] Fifteen subjects with documented ascites due to cirrhosis, but not type 1 or 2 HRS, will be administered continuous low doses (2.0 to 3.0 mg escalating every 24 hours) via a portable infusion pump terlipressin. These patients are expected to experience a reduction in ascites severity and ascites fluid accumulation over the course of 1 to 28 days of treatment. This approach is also expected to reduce the number of puncture procedures required to remove ascitic fluid over a 28-day period compared to a 28-day cycle prior to treatment initiation, and some patients should avoid punctures altogether. In addition, the mean volume of fluid withdrawn after initiation of continuous infusion pump terlipressin therapy should be significantly less than before initiation of therapy. Furthermore, improvements in the patient's health status can be safely achieved without serious side effects. Ther...
Embodiment 2
[0025] Example 2 : Treatment of ascites with continuous infusion pump terlipressin
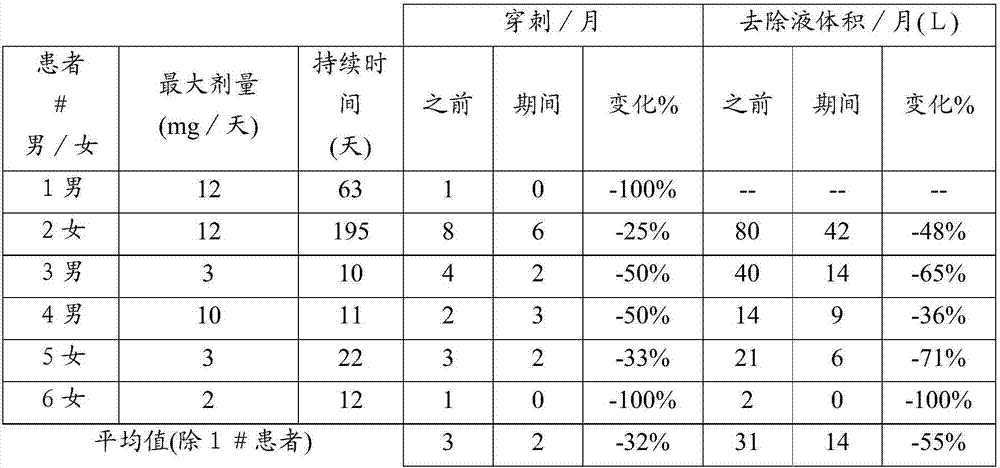
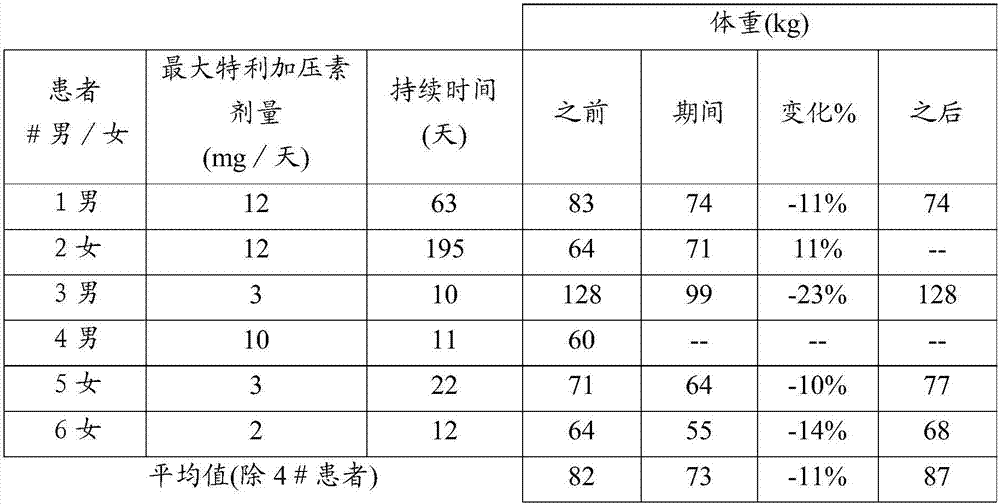
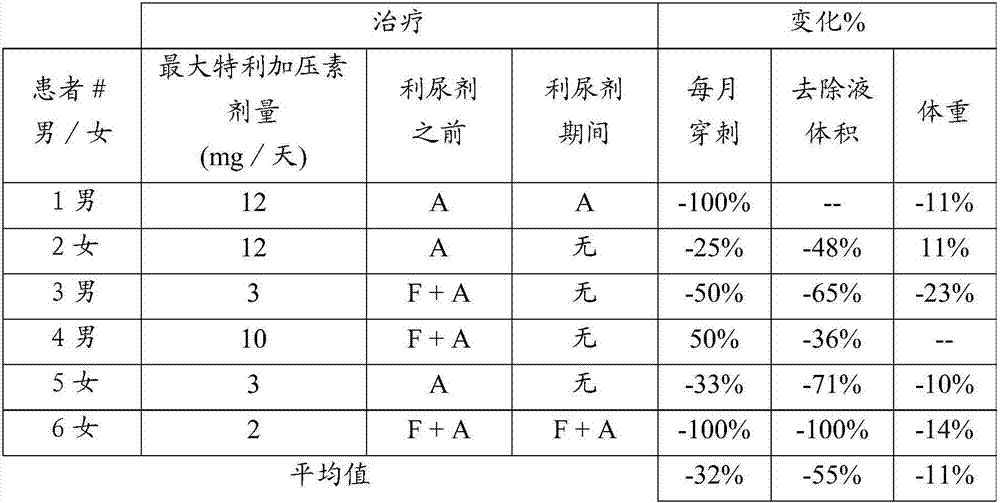
[0026] Ascites improvement was assessed in six HRS patients treated with continuous infusion terlipressin. All 6 patients had diuretic-refractory or refractory ascites (5 of 6 with hyponatremia). Patients were evaluated before, during and after treatment with the following parameters: number of puncture procedures per month, volume of ascites removed, body weight, serum sodium, urinary sodium excretion, serum creatinine, serum urea, and whether diuretics were included in the course of treatment middle. None of the six patients had a complete dataset for all parameters. The effect of continuous infusion of terlipressin on each parameter is shown in Tables 1-7.
[0027] Reduced puncture frequency and fluid volume during treatment
[0028] The average number of monthly paracentesis procedures decreased from three before starting continuous infusion therapy to two during treatment, and the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com