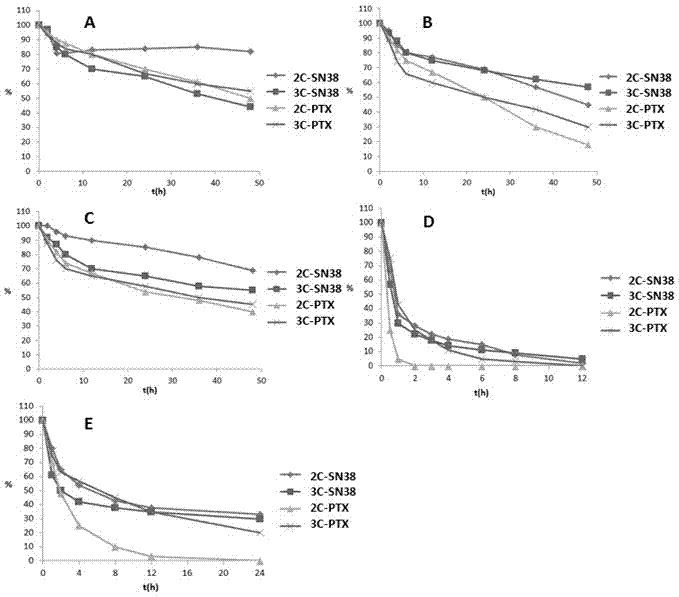
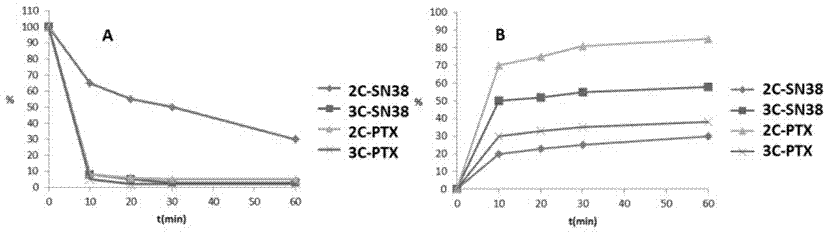
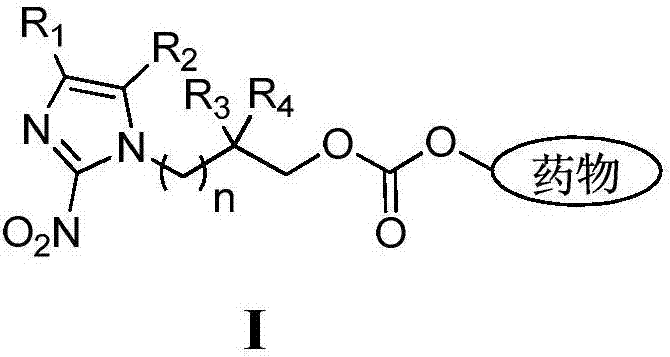
Hypoxia-activated prodrug based on 2-nitroimidazole-1-alkanol
A technology based on nitroimidazole and nitroimidazole, which is applied in the field of hypoxia-activated prodrugs and their synthesis of 2-nitroimidazole-1-alkanols, which can solve the problems of incomplete transformation of the original drug form of the drug , to achieve the effects of enhancing curative effect and bioavailability, improving targeting and increasing selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of compound 3
[0046]
[0047]Cesium carbonate (5.8g, 17.70mmol) was added to the N,N-dimethylformamide solution of compound 1 (1g, 8.85mmol), stirred at room temperature for 5 minutes, and compound 2 (1.4g, 9.73mmol) was added to the system ), the reaction mixture was reacted at 50°C for 12 hours, the reaction system was concentrated by rotary evaporation with an oil pump, and the concentrate was separated and purified by column chromatography (dichloromethane:methanol=60:1) to obtain a light yellow solid (1.3g, 88%). 1 H NMR (400MHz, CDCl 3 )δ7.17(s,1H),7.14(s,1H),4.59(t,J=6.9Hz,2H),3.68(t,J=5.7Hz,2H),2.16-2.05(m,2H), 1.78(s,1H). 13 C NMR (101MHz, DMSO) δ144.7, 128.0, 127.9, 57.6, 47.1, 32.4. MS (ESI) m / z = 172.06 [M+H] + .
Embodiment 2
[0049] Preparation of compound 5
[0050]
[0051] Under ice-cooling, tert-butyldimethylsilyl chloride (6.4g, 42mmol) was added in batches to a dichloromethane solution of compound 4 (5g, 40mmol) and imidazole (5.5g, 80mmol), and reacted at room temperature for 2 hours. The system was diluted with dichloromethane, washed with saturated aqueous sodium carbonate, washed with water, washed with saturated aqueous sodium chloride, the organic phase was dried over anhydrous sodium sulfate, filtered, and spin-dried to obtain a yellow oil (6.7g, 70%). 1 H NMR (400MHz, CDCl 3 )δ3.88(t,J=6.5Hz,2H),3.38(t,J=6.5Hz,2H),0.91(s,9H),0.08(s,6H).MS(ESI)m / z=238.04 [M+H] + .
Embodiment 3
[0053] Preparation of compound 6
[0054]
[0055] Add cesium carbonate (7g, 21.24mmol) to the N,N-dimethylformamide solution of compound 1 (1.2g, 10.62mmol), and add compound 5 (2.8g, 11.68mmol) under stirring, and the reaction mixture is 60°C After 12 hours of reaction, the reaction mixture was quenched with water, extracted with ethyl acetate, the organic phase was washed with water and saturated aqueous sodium chloride solution, and the organic phase was spin-dried to obtain a yellow solid (2 g, 70%). 1 H NMR (400MHz, CDCl 3 )δ7.38(s,1H),7.25(s,1H),4.67(t,J=4.9Hz,2H),4.07(t,J=4.9Hz,2H),0.95(s,9H),0.06( s,6H).MS(ESI)m / z=272.83[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com