Application of compound to preparation of medicament for cathepsin-K-targeted disease
A cathepsin and compound technology, applied in metabolic diseases, bone diseases, digestive system, etc., can solve the problems of inactive site inhibitors, side effects of active site inhibitors, dysfunction of thyroxine release, etc., and achieve inhibition Effects of human osteoclast activity, increasing bone density, improving bone structural characteristics and physiological state
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
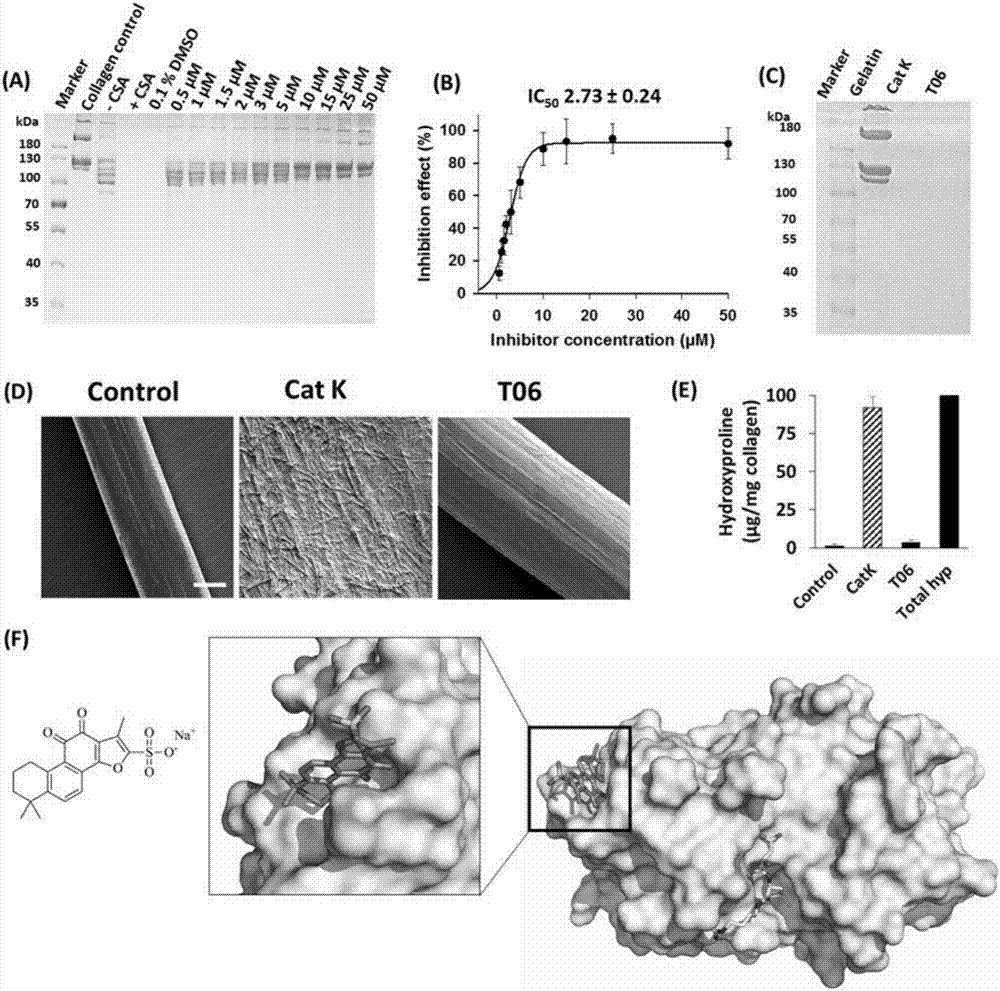
[0038] Example 1: Screening and Confirmation of Novel Inactive Site Inhibitors of Cathepsin K
[0039] Seven monomer compounds in Danshen, including type I structure danshenneoquinone D, type II structure dihydrotanshinone I, tanshinone I, tanshinone IIA, cryptotanshinone, sodium tanshinone IIA sulfonate and type III structure table salvianolactone were investigated. Screening for active site inhibitors. The structure is as follows:
[0040]
[0041] 1 Experimental materials
[0042] 1.1 Medicinal materials and reagents
[0043]Monomer compounds tanshinone D, dihydrotanshinone I, tanshinone I, tanshinone IIA, cryptotanshinone, tanshinone IIA sodium sulfonate and epidanshinone cryptospironolactone were purchased from Chemfaces; Benzyloxycarbonyl-Phe-Arg-7-amido-4- Methylcoumarin (Z-FR-MCA) was purchased from Japan WAKO Company; chondroitin sulfate A (C4-S), E-64 (L-3-carboxy-trans-2-3-epoxypropionyl-leucylamido-(4guanidino)-butane) Pepsin and pepsin were purchased from S...
Embodiment 2
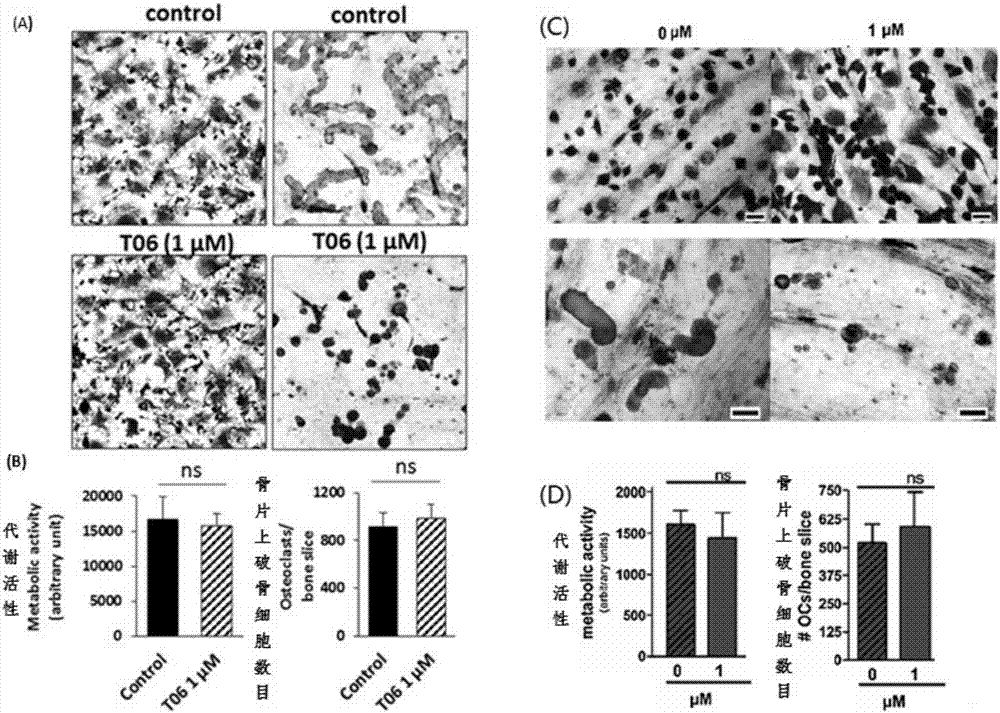
[0064] Example 2: Effect of Cathepsin K Inhibitor T06 on Osteoclast Bone Resorption
[0065] Osteoclasts express a rich intracytoplasmic enzyme system, and a series of marker proteins are produced during the differentiation of osteoclast precursor cells to mature osteoclasts, which can be used as markers for the identification of osteoclasts and their differentiation stages. Among them, tartrate-resistant acid phosphatase (TRAP) expressed by differentiated osteoclast precursor cells is considered to be a marker enzyme of osteoclasts. In this experiment, bone marrow-induced osteoclast model was used to investigate the effects of T02 and T06 on osteoclast differentiation and TRAP activity.
[0066] 1 Experimental materials
[0067] 1.1 Medicinal materials and reagents
[0068] Animals: 5 littermate SD mice born 1-2 days old, male or female, weighing 7-8 g, provided by the Experimental Animal Center of Second Military Medical University.
[0069] Tanshinone IIA sodium sulfonat...
Embodiment 3
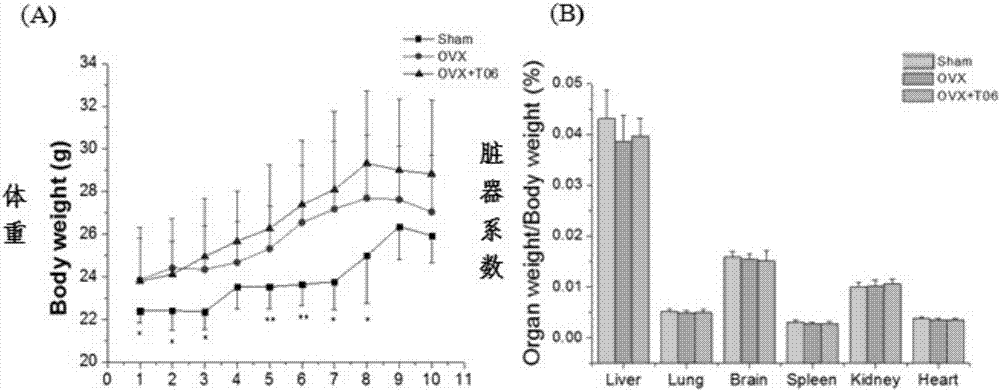
[0083] Example 3: Effects of Cathepsin K Inhibitor T06 on Ovariectomized Osteoporotic Mice
[0084] 1 Experimental materials
[0085] Animals: C57BL6 mice were provided by the University of British Columbia, Canada. Animals were reared in separate cages (3-4 animals / cage) in an air-conditioned greenhouse at a temperature of 21±2°C and a humidity of 40-60%. They were fed with pellet feed and had free access to water.
[0086] 2 Experimental methods
[0087] Animal grouping: 30 female C57BL6 mice (18-22g) were randomly divided into 3 groups according to body weight, 10 in each group, including sham operation group (SHAM, intragastric administration of normal saline 10ml / kg), osteoporosis model group (OVX, give normal saline 10ml / kg) and T06 (40mg / kg / d) 3 groups. After recovering for 5 days, the drug was given continuously by intragastric administration for 12 weeks.
[0088] Modeling method: Isoflurane anesthesia on the operating table, two 0.5 cm incisions were made on the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com