Synthesis of related substances of 5,6,4'-trihydroxyflavone-7-0-d-glucuronic acid and its preparation method and application
A technology of glucuronic acid and trihydroxyflavone, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of no research and achieve the effects of simple operation, high product purity and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) Preparation of Compound III
[0052] 20g (27.4mmol) of 5,6,4'-triacetoxyflavone-7-O-D-methyl triacetoxyglucuronate was dissolved in 200mL of toluene, and then slowly added dropwise to 1,8-diazepine under stirring Dicycloundec-7-ene (DBU) 4.1g (27mmol), stirred at 30°C for about 8 hours to complete the reaction. About 500 ml of ethyl acetate was added to the reaction mixture with stirring, and washed twice with 500 ml of water. After the water layer was separated, an appropriate amount of anhydrous sodium sulfate was added to the organic layer to dry, and evaporated to dryness under reduced pressure. Silica gel column chromatography and elution with petroleum ether: ethyl acetate = 5:1 (v / v) eluent gave 15 g of white solid compound III with a chromatographic purity of over 98.00% and a yield of 82%.
[0053] 1 H-NMR (400MHz, DMS0), δ (ppm): 7.95 (2H, d, J = 8.6Hz), 7.51 (1H, s), 7.32 (2H, d, J = 8.6Hz), 6.78 (1H, s ),6.15(1H,d,J=7.8Hz),5.57(1H,d,J=10Hz), 5.38(1H,...
Embodiment 2
[0060] (1) Preparation of Compound III
[0061] 20g (27.4mmol) of 5,6,4'-triacetoxyflavone-7-O-D-methyl triacetoxyglucuronate was dissolved in 200mL of dichloromethane, then slowly added 4-dimethylaminopyridine under stirring (DMAP) 1.7g (13.7mmol, 0.5eq), stirred at 25°C for about 10 hours and the reaction was complete. About 300 ml of ethyl acetate was added to the reaction mixture with stirring, and it was washed twice with 500 ml of water. After the water layer was separated, an appropriate amount of anhydrous sodium sulfate was added to the organic layer to dry, and evaporated to dryness under reduced pressure. Silica gel column chromatography and elution with petroleum ether: ethyl acetate = 5:1 (v / v) eluent gave 14.3 g of white solid compound III with a chromatographic purity of over 98.00% and a yield of 78%. The NMR identification result is consistent with the result of Example 1.
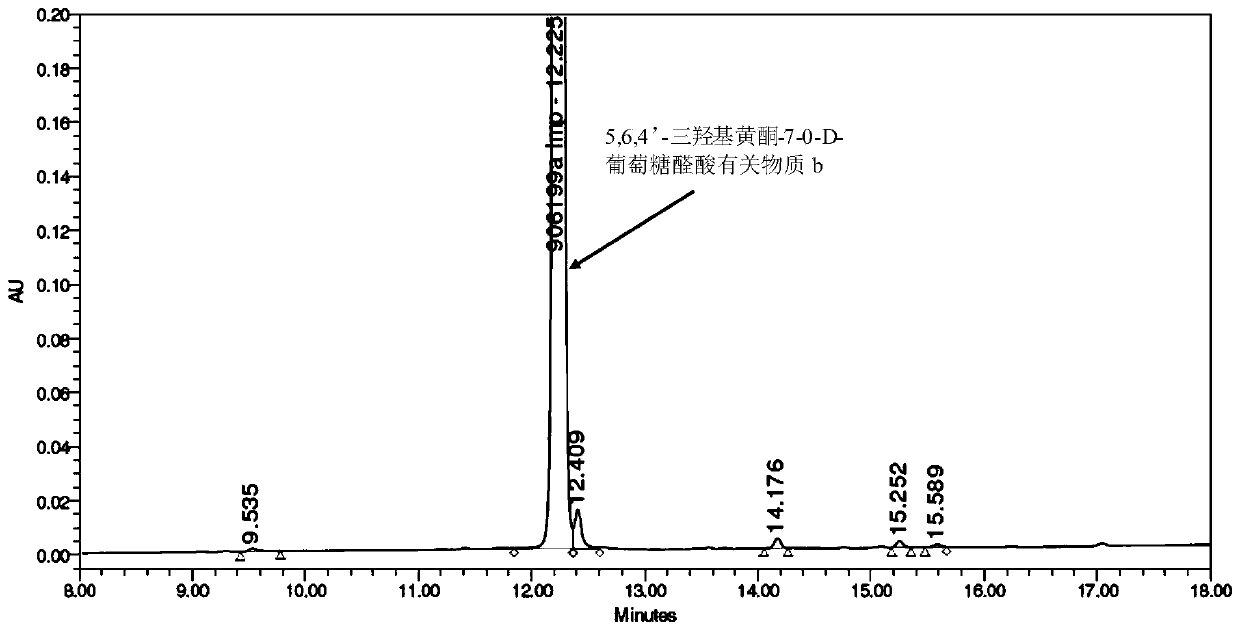
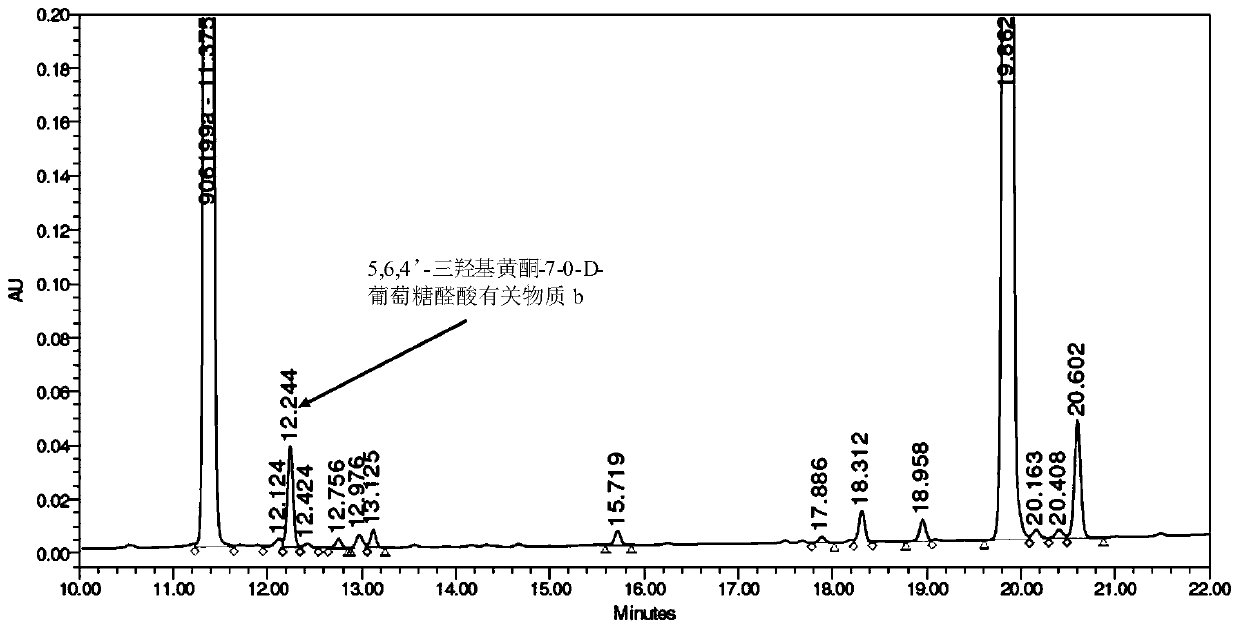
[0062] (2) Preparation of 5,6,4'-trihydroxyflavone-7-0-D-glucuronic acid related su...
Embodiment 3
[0065] (1) Preparation of Compound III
[0066] 20g (27.4mmol) of 5,6,4'-triacetoxyflavone-7-O-D-methyl triacetoxyglucuronate was dissolved in 200mL of tetrahydrofuran, and then 6.5g (82.2mmol) of pyridine was added dropwise under stirring, The reaction was stirred at 25°C for about 6 hours and the reaction was complete. About 200ml of ethyl acetate was added to the reaction mixture under stirring, and washed twice with 500°C water. After the water layer was separated, an appropriate amount of anhydrous sodium sulfate was added to the organic layer to dry, and evaporated to dryness under reduced pressure. Silica gel column chromatography and elution with petroleum ether: ethyl acetate = 5:1 (v / v) eluent gave 14.7 g of white solid compound III with a chromatographic purity of over 98.00% and a yield of 80%. The NMR identification result is consistent with the result of Example 1.
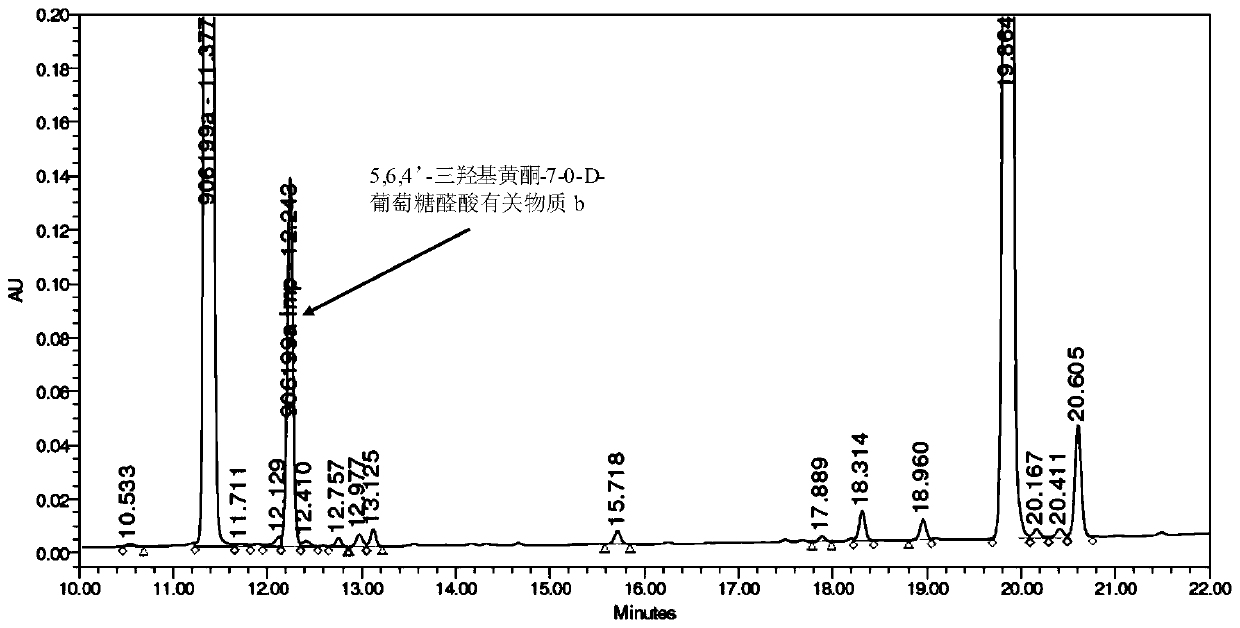
[0067] (2) Preparation of 5,6,4'-trihydroxyflavone-7-0-D-glucuronic acid related substance b ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com