A kind of medicine for lowering blood fat and preparation method thereof
A technology of medicine and pharmacy, which is applied in the field of preparing medicines for lowering blood lipids and/or treating atherosclerosis, and can solve the problems of affecting bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1: 1-((3-methoxy-4-methyl-6-oxo-4,5,6,7-tetrahydro-2H-pyrazol[3,4-b]pyridine-2- Base) methyl) -3- (piperidin-1-yl) urea (compound I)
[0075]
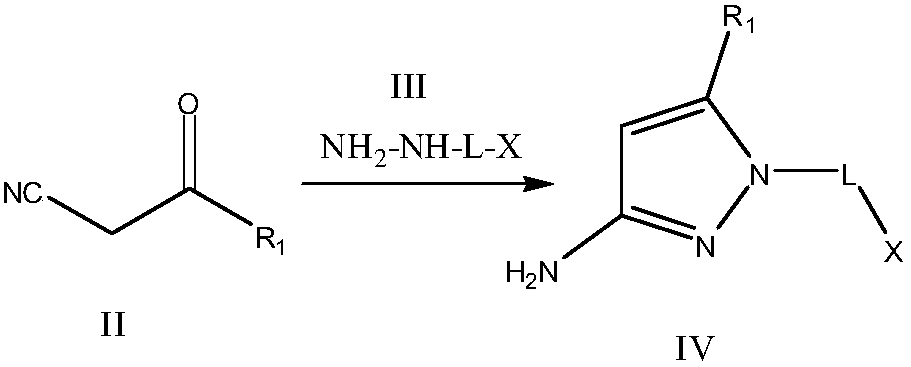
[0076]Step 1: Bromomethylhydrazine (2.46 g, 20.0 mmol) was added to a solution of methyl 2-cyanoacetate (1.98 g, 20.0 mmol) in ethanol (100 mL), and the mixture was stirred at 50° C. for 2 hours. The reaction solution was concentrated under reduced pressure, and the obtained residue was separated into an organic layer and an aqueous layer by adding saturated aqueous sodium bicarbonate solution and ethyl acetate. The organic layer was washed with brine and dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The obtained residue was recrystallized with chloroform to obtain 1-(bromomethyl)-5-methoxy-1H-pyrazol-3-amine 2.93 g, yield: 72%, content 99%. ESI-MS: 205.99[M+H] + .
[0077] Step 2: Add acetaldehyde methyl hemiacetal (0.76g, 20.mmol) to the 1-(bromomethyl)-5-methoxy-...
Embodiment 2
[0082] Example 2: 1-((3-((2H-1,2,3-triazol-2-yl)methyl)-4-(fluoromethyl)-6-oxo-4,5,6, 7-tetrahydro-2H-pyrazol[3,4-b]pyridin-2-yl)methyl)-3-thiomorpholinourea (Compound II)
[0083]
[0084] According to the method of Example 1, with 3-oxo-4-(2H-1,2,3-triazol-2-yl)butyronitrile instead of methyl 2-cyanoacetate, 1-fluoroacetaldehyde methyl semi Acetal in place of acetaldehyde methyl hemiacetal and 1-(thiomorpholin-4-yl)urea in place of 1-(piperazin-1-yl)urea afforded the title compound as a pale yellow solid, the total product of three steps rate of 38%.
[0085] ESI-MS: 424.16[M+H] +
[0086] Elemental analysis: theoretical value / measured value, C(45.38 / 45.48), H(5.24 / 5.29), F(4.49 / 4.53), N(29.77 / 29.64), O(7.56 / 7.51), S(7.57 / 7.55)
[0087] 1 H NMR(400MHz,DMSO-D6)δ8.04(s,1H),7.62(d,2H),6.03(s,1H),5.97(s,1H),5.61(s,2H),4.99(s, 2H), 4.55(q, 1H), 4.25(q, 1H), 3.25(m, 1H), 2.91(t, 4H), 2.72(t, 4H), 2.61(q, 1H), 2.31(q, 1H ).
Embodiment 3
[0088] Example 3: 1-(3-chloropyridin-2-yl)-3-((3-(2-ethoxyethyl)-6-oxo-4-(trifluoromethyl)-4,5 ,6,7-tetrahydro-2H-pyrazol[3,4-b]pyridin-2-yl)methyl)urea (compound III)
[0089]
[0090] According to the method of Example 1, replace methyl 2-cyanoacetate with 5-ethoxyl-3-oxovaleronitrile, trifluoroacetaldehyde methyl hemiacetal instead of acetaldehyde methyl hemiacetal, use 1- (3-Chloro-pyridin-2-yl)urea in place of 1-(piperazin-1-yl)urea afforded the title compound as a white solid in 35% overall yield over three steps.
[0091] ESI-MS: 461.12[M+H] +
[0092] Elemental analysis: theoretical value / measured value, C(46.91 / 46.78), H(4.37 / 4.31), Cl(7.69 / 7.78), F(12.37 / 12.47), N(18.24 / 18.34), O(10.42 / 7.32)
[0093] 1 H NMR (400MHz, DMSO-D6) δ9.54(s,1H),8.04(s,1H),8.12(d,1H),7.62(d,1H),6.81(q,1H),6.08(s, 1H),5.63(s,2H),3.89(q,1H),3.55(t,2H),3.47(q,2H),2.75(t,2H),2.61(q,1H),2.32(q,1H ), 1.11(t,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com