A kind of preparation method of hydrogen production catalyst by steam reforming of methane
A methane water vapor, reforming hydrogen production technology, applied in the direction of catalyst activation/preparation, molecular sieve catalysts, chemical instruments and methods, etc., can solve the problems of high cost, high price, easy carbon deposition of catalysts, etc., to reduce the amount of metal, Conducive to industrial scale-up and reduce the effect of concentration difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
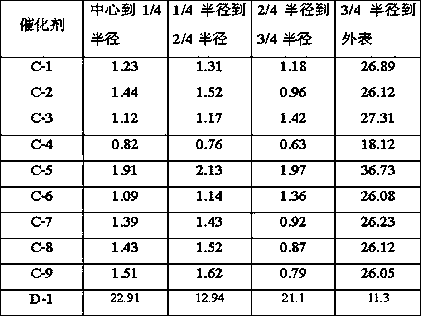
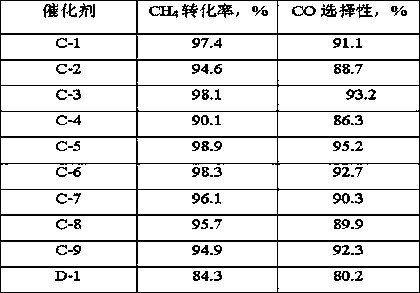
[0026] Weigh 2.97g of nickel nitrate and dissolve it in 14mL of deionized water to obtain solution A; use equal volume impregnation method to load on 15.2g of alumina carrier (pore volume is 0.73mL / g, specific surface area is 253m 2 / g, strip shape, equivalent diameter 1.5mm), impregnated at room temperature for 2h, aged for 4h, dried at 80°C for 12h, and calcined at 700°C for 4h to prepare catalyst precursor B. The supported Ni was 3% by weight of the final catalyst %; Catalyst precursor B is activated in a mixed atmosphere containing hydrogen, the volume content of hydrogen in the mixed gas is 80%, the reduction condition is 450°C, 0.2MPa (absolute pressure), and the reduction time is 4h; 6.41g magnesium nitrate is dissolved in 16mL In deionized water, solution C was obtained, and the mass fraction of 4 times its mass was mixed uniformly with 40% furfural aqueous solution, and then joined in the autoclave together with the catalyst precursor B after reduction and activation; 1...
Embodiment 2
[0028] Weigh 2.97g of nickel nitrate and dissolve it in 14mL of deionized water to obtain solution A; use equal volume impregnation method to load on 15.2g of silica carrier (pore volume is 0.97mL / g, specific surface area is 372m 2 / g, spherical shape, equivalent diameter 0.5mm), impregnated at room temperature for 2h, aged for 4h, dried at 80°C for 12h, and calcined at 700°C for 4h to prepare catalyst precursor B. The supported Ni was 3% of the final catalyst based on the weight of the element ; Catalyst precursor B was activated in a mixed atmosphere containing hydrogen, the volume content of hydrogen in the mixed gas was 80%, the reduction conditions were 450°C, 0.2MPa (absolute pressure), and the reduction time was 4h; 6.41g of magnesium nitrate was dissolved in 16mL to In deionized water, obtain solution C, and its 4 times mass fraction is the furfural aqueous solution mixing uniformly of 40%, then joins in the autoclave together with the catalyst precursor B after reducti...
Embodiment 3
[0030] Weigh 2.97g of nickel nitrate and dissolve it in 14mL of deionized water to obtain solution A; use equal volume impregnation method to load on 15.2g of SBA-15 carrier (pore volume is 1.23mL / g, specific surface area is 701m 2 / g, strip shape, equivalent diameter 1.5mm), impregnated at room temperature for 2h, aged for 4h, dried at 80°C for 12h, and calcined at 700°C for 4h to prepare catalyst precursor B. The supported Ni was 3% by weight of the final catalyst %; Catalyst precursor B is activated in a mixed atmosphere containing hydrogen, the volume content of hydrogen in the mixed gas is 80%, the reduction condition is 450°C, 0.2MPa (absolute pressure), and the reduction time is 4h; 6.41g magnesium nitrate is dissolved in 16mL In deionized water, solution C was obtained, and the mass fraction of 4 times its mass was mixed uniformly with 40% furfural aqueous solution, and then joined in the autoclave together with the catalyst precursor B after reduction and activation; 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com