Aldehyde group compounds, as well as preparation method and application thereof
A technology of compounds, aldehyde groups, applied in the fields of medicinal chemistry and pharmacotherapeutics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
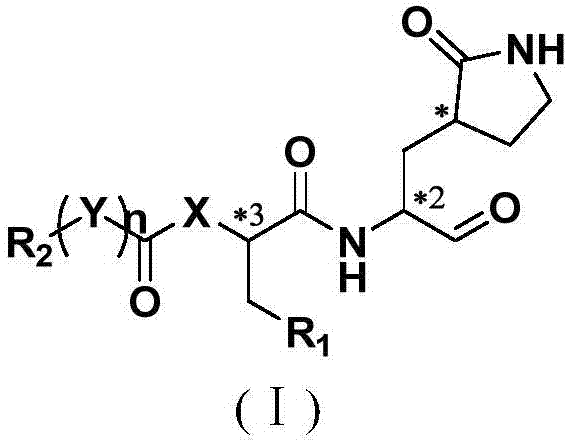
[0090] The preparation of formula (I) compound
[0091] The present invention also provides a synthetic method for a compound of general formula I, specifically, said compound of formula I is prepared by the following processes:
[0092]
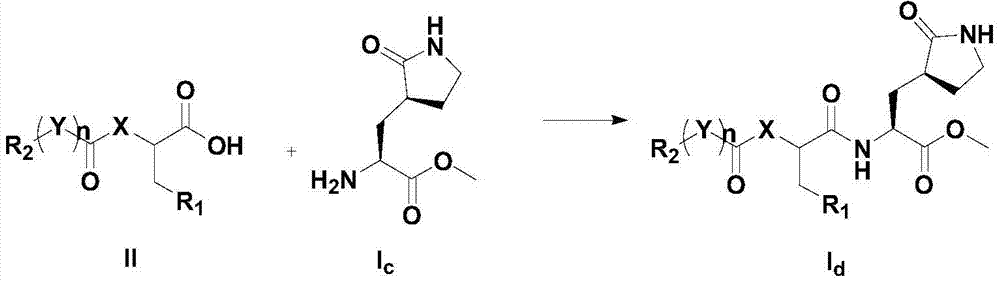
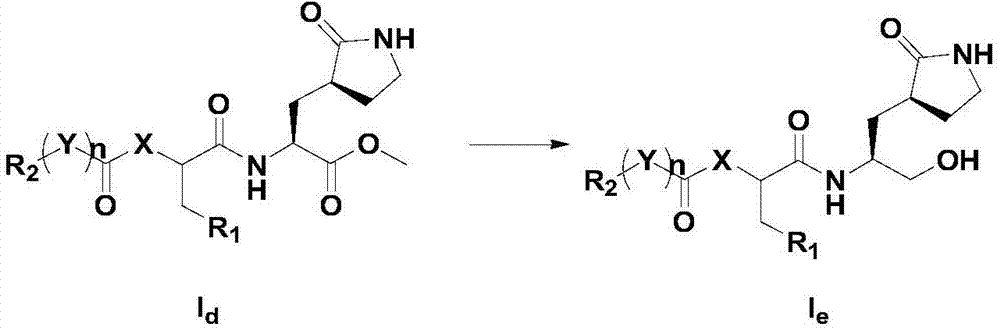
[0093] Step a: Dissolve dimethyl tert-butoxycarbonyl glutamate in a solvent, add alkali at -78°C and stir, then add bromoacetonitrile, and continue stirring to obtain compound I a , the solvent is tetrahydrofuran or dioxane; the base is lithium hexamethyldisilazide or lithium diisopropylamide;
[0094] Step b: put I a Dissolve in a solvent, add a catalytic amount of platinum dioxide, stir until the raw materials are completely reacted, filter, add alkali, and stir under reflux to obtain compound I b ; The alkali is sodium carbonate or sodium acetate; The solvent is a mixed solvent of methanol and chloroform;
[0095] Step c: put I b Dissolve in the solvent, stir until the reaction is complete, and spin the solvent to obtain compound I...
Embodiment 1
[0121] Embodiment 1: the synthesis of compound 1
[0122]
[0123] synthetic route:
[0124]
[0125] Synthesis of Compound 1-3:
[0126] Under the protection of argon, N-tert-butoxycarbonyl-L-glutamic acid dimethyl ester (1-1) (6 g, 21.8 mmol) was dissolved in 60 mL of anhydrous tetrahydrofuran, and LiHMDS ( 1M in THF) in tetrahydrofuran (47mL, 47mmol), the temperature was kept stable at -78°C during the dropwise addition for about 1 hour. After the dropping, the mixture was stirred at -78°C for 1 hour. Bromoacetonitrile (2.79 g, 23.3 mmol) was dissolved in 20 ml of tetrahydrofuran, and then the solution was slowly dropped into the reaction system, and the dropping process lasted for 1 to 2 hours. The temperature was controlled at -78°C, and the reaction was continued for 20 hours. THL monitoring (alkaline potassium permanganate color development) After the reaction is complete, add 3 mL of methanol and 22.7 mL of a mixed solution of glacial acetic acid and tetrahyd...
Embodiment 2
[0149] Embodiment 2: the synthesis of compound 2
[0150]
[0151] Compound 1-11 was used to replace acid 1-13 in Example 1, and the synthesis method was referred to the synthesis of compound 1 to obtain compound 2. 1 H NMR (400MHz, CDCl3): δ9.32-9.26(m,1H),7.81(m,1H),7.54-7.18(m,8H),7.13-6.91(m,1H),6.44-6.08(m, 1H),5.15-4.90(m,1H),4.65(m,2H),4.47-4.04(m,2H),3.45-3.03(m,4H),2.54-2.30(m,1H),2.24-2.16( m,1H),2.12-1.54(m,3H).ESI-MSm / z 496.15[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com