Applications of calcium amino acid chelate in preparation of percutaneous absorption preparation
An amino acid and chelated calcium technology, which is applied in skin care preparations, applications, skin diseases, etc., can solve problems such as inability to replenish calcium ions in the epidermis, difficulties in stabilizing calcium ions in the epidermis, and difficulty in absorbing calcium ions, so as to achieve enhanced utilization efficiency, Excellent transdermal absorption effect, effect of increasing calcium storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
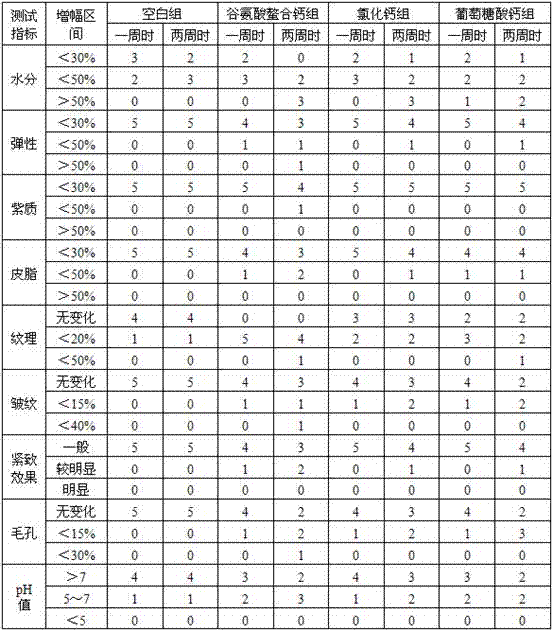
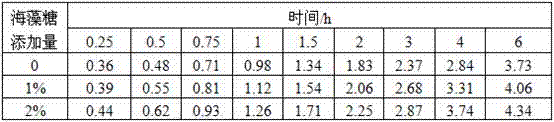
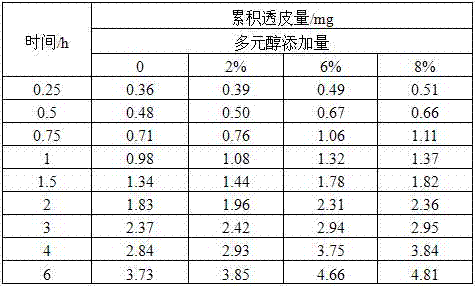
Examples
Embodiment 1
[0043] Preparation of amino acid chelated calcium: In this experiment, glutamic acid was used as a ligand, and calcium chloride, calcium carbonate, calcium phosphate, calcium hydrogen phosphate, calcium silicate, calcium lactate, calcium propionate, calcium citrate, glucose A kind of in acid calcium is used as the calcium source that provides central ion calcium to prepare amino acid chelated calcium, to do further experimental research, specifically as follows:
[0044] 1. Preparation of calcium glutamate chelate
[0045] 1) Weigh 45 g of glutamic acid, dissolve it in a three-necked flask with 150 ml of deionized water, and heat it in a water bath to a constant temperature of 80°C until glutamic acid is completely dissolved;
[0046] 2) Weigh 20g of calcium lactate, completely dissolve in enough water, and adjust the pH to 7.8;
[0047] 3) Slowly add the calcium lactate solution prepared in 2) to the glutamic acid solution in 1) drop by drop, stir gently while adding the cal...
Embodiment 2
[0059] In this experiment, glutamic acid and glycine were used as ligands, and one of calcium chloride, calcium carbonate, calcium phosphate, calcium hydrogen phosphate, calcium silicate, calcium lactate, calcium propionate, calcium citrate, and calcium gluconate was randomly selected. As a calcium source that provides central ion calcium, prepare amino acid chelated calcium with two different amino acid ligands in the same molecule for further experimental research, as follows:
[0060] 1. Preparation of polyamino acid chelated calcium
[0061] 1) Weigh 40g of calcium lactate, dissolve it completely in sufficient water, adjust the pH to 7.8, add it to the reactor, and heat it in a water bath to a constant temperature of 80°C;
[0062] 2) Weigh 40 g of glycine and glutamic acid respectively, dissolve them in 150 ml of deionized water, heat in a water bath to a constant temperature of 80°C, and prepare glycine aqueous solution and glutamic acid aqueous solution until glycine an...
Embodiment 3
[0069] In this experiment, glutamic acid was used as a ligand, and any one of calcium chloride, calcium carbonate, calcium phosphate, calcium hydrogen phosphate, calcium silicate, calcium lactate, calcium propionate, calcium citrate, and calcium gluconate was selected as the ligand. The calcium source of the central ion calcium is provided, and the glutamic acid chelated calcium with different chelation ratios is prepared.
[0070] Preparation of calcium glutamate chelate with a chelation ratio of glutamate:calcium = 2:1
[0071] 1) Weigh 45 g of glutamic acid, dissolve it in a three-necked flask with 150 ml of deionized water, and heat it in a water bath to a constant temperature of 80°C until glutamic acid is completely dissolved;
[0072] 2) Weigh 20g of calcium lactate, completely dissolve in enough water, and adjust the pH to 7.8;
[0073] 3) Slowly add the calcium lactate solution prepared in 2) to the glutamic acid solution in 1) drop by drop, stir gently while adding,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com