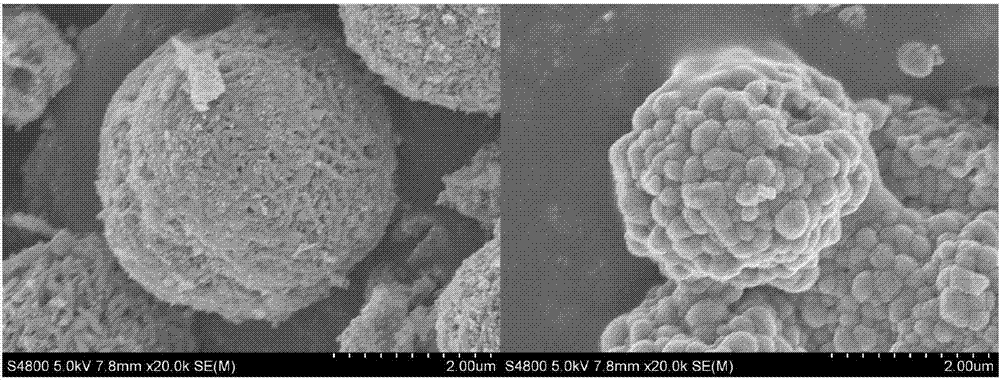
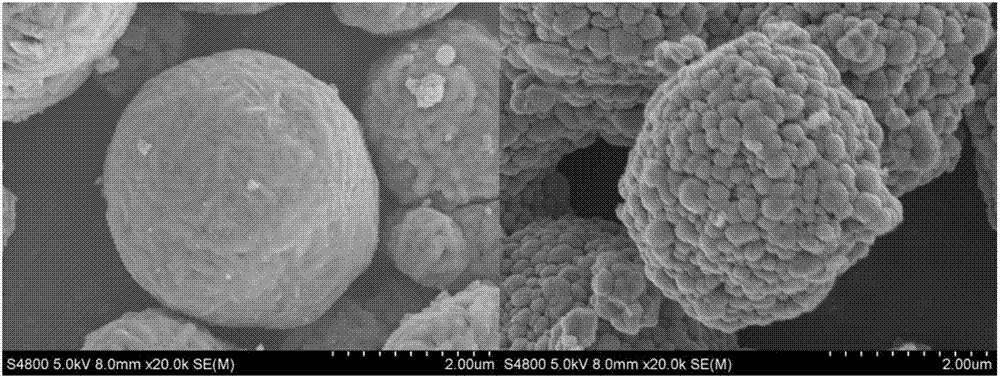
High-nickel material coated with Li2TiO3 on surface and doped with Ti on surface layer and preparation method
A surface-coated, high-nickel technology, applied in electrical components, electrochemical generators, battery electrodes, etc., can solve the problems of high-nickel material deterioration of cycle performance and safety performance, increased impedance, and increased electrochemical engineering energy consumption. Achieve the effect of improving electrochemical performance and structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Step 1. Take by weighing 47.313g NiSO in the ratio of molar ratio Ni:Co=0.9:0.1 4 ·6H 2 O, 5.622gCoSO 4 ·7H 2 O soluble inorganic salts are made into nickel and cobalt ion total concentration is 2mol L with 100mL deionized water -119.2g NaOH and 32.3mL ammonia water are mixed with deionized water according to the molar ratio of 1.5 so that the NaOH molar concentration is 0.4mol L -1 Mix the alkaline solution.
[0039] Step 2. Use 100mL deionized water as the reaction base solution, start stirring and heating to stabilize the temperature at 45°C, continue stirring and temperature until the end of the reaction, the stirring rate is 600r / min, add ammonia water to adjust the pH to about 11.5. Slowly and uniformly introduce the mixed salt solution and mixed alkali solution into the reaction kettle, and control the pH to be stable at about 11.5 during introduction. The feeding time is 24h, and the stirring is continued for 24h after the feeding is finished. After the re...
Embodiment 2
[0045] Step 1. Take by weighing 47.313g NiSO in the ratio of molar ratio Ni:Co=0.9:0.1 4 ·6H 2 O, 5.622gCoSO 4 ·7H 2 O soluble inorganic salts are made into 2mol L with 100mL deionized water -1 19.2g NaOH and 32.3mL ammonia water are mixed with deionized water according to the molar ratio of 1.5 so that the NaOH molar concentration is 0.4mol L -1 Mix the alkaline solution.
[0046] Step 2. Use 100mL deionized water as the reaction base solution, start stirring and heating to stabilize the temperature at 55°C, continue stirring and temperature until the end of the reaction, the stirring rate is 600r / min, add ammonia water to adjust the pH to about 11.5. Slowly and uniformly introduce the mixed salt solution and the mixed alkali solution into the reaction kettle, and control the pH to be stable at 11.5 during introduction. The feeding time is 24h, and the stirring is continued for 18h after the feeding is finished. After the reaction is completed, the precipitate is filter...
Embodiment 3
[0052] Step 1. Take by weighing 47.313g NiSO in the ratio of molar ratio Ni:Co=0.9:0.1 4 ·6H 2 O, 5.622gCoSO 4 ·7H 2 O soluble inorganic salts are made into 2mol L with 100mL deionized water -1 mixed salt solution, and then use deionized water to prepare NaOH and ammonia water according to the molar ratio of 1.5 to make the NaOH molar concentration 0.4mol L -1 Mix the alkaline solution.
[0053] Step 2. Use 100mL deionized water as the reaction base solution, start stirring and heating to stabilize the temperature at 65°C, the stirring rate is 600r / min, add 3-5 drops of ammonia water to adjust the pH to about 11.5. Slowly and uniformly introduce the mixed salt solution and the mixed alkali solution into the reaction kettle, and control the pH to be stable at 11.5 during introduction. The feeding time is 24h, and the stirring is continued for 12h after the feeding is finished. After the reaction is completed, the precipitate is filtered, washed, and dried to obtain a high...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com