Method for continuous flow synthesis of phenol-based compound
A technology for phenolic compounds and aniline compounds, applied in the field of continuous flow synthesis of phenolic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
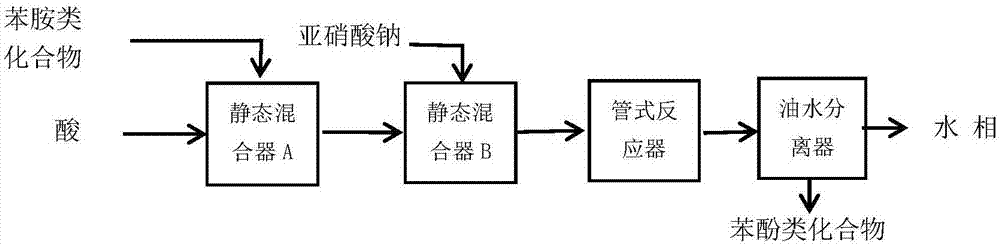
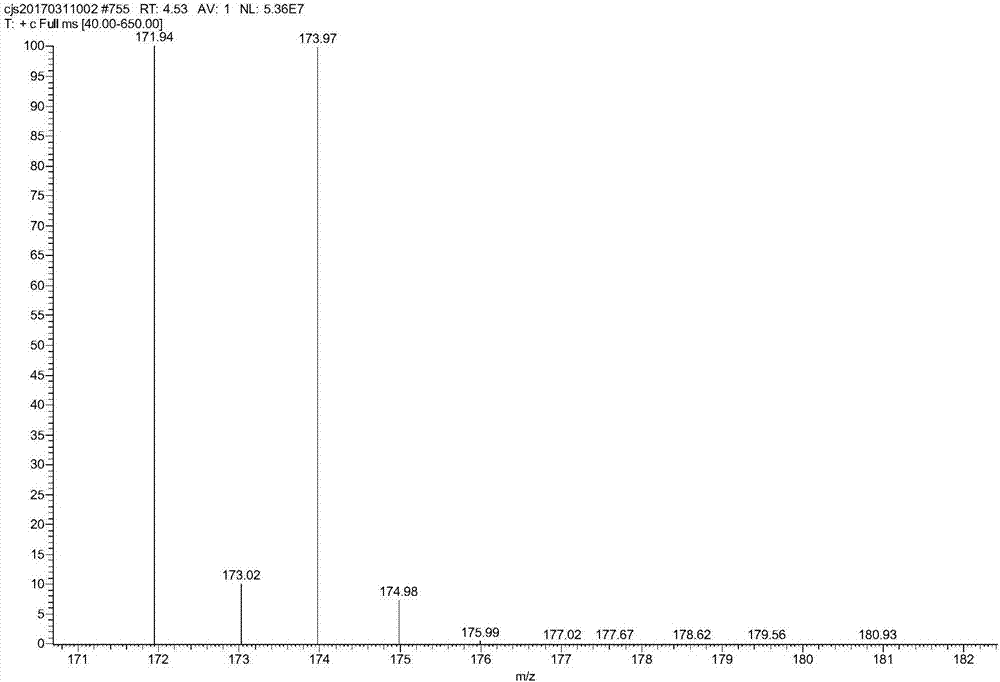
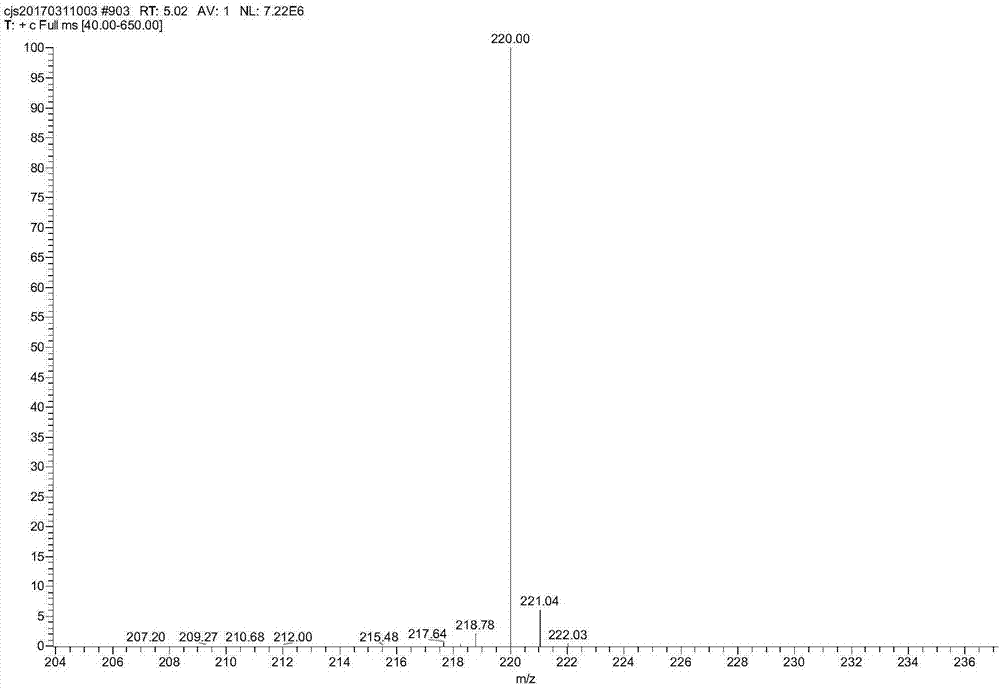
[0032] At room temperature, the sulfuric acid solution with a mass percent concentration of 25% is pumped into the straight static mixer A with an electromagnetic metering pump at a speed of 49.9g / min, and then m-trifluoromethylaniline is pumped at a rate of 4.1g / min with an electromagnetic metering pump. The speed is pumped into the straight static mixer A, the temperature of the heat transfer oil of the straight static mixer A is set to 12.5 °C, and the sodium nitrite solution with a concentration of 30% by mass is used at a rate of 6 g / min with a Hanbon-NP7010C high-efficiency liquid phase pump. The speed is pumped into the heart-shaped static mixer B, and the temperature of the heat transfer oil in the heart-shaped static mixer B is set at 12.5°C, and the diazonium salt solution is obtained after the reaction. Set the temperature of the heat transfer oil in the heart-shaped tubular reactor to 88°C, and at the same time, use a Hanbon-NP7010C high-efficiency liquid phase pump...
Embodiment 2
[0034] At room temperature, the sulfuric acid solution with a mass percent concentration of 25% is pumped into the straight static mixer A with an electromagnetic metering pump at a speed of 49.9g / min, and then m-trifluoromethylaniline is pumped at a rate of 4.1g / min with an electromagnetic metering pump. The speed is pumped into the straight static mixer A, the temperature of the heat transfer oil in the straight static mixer A is set to 0°C, and the sodium nitrite solution with a mass percentage concentration of 30% is pumped at a rate of 6g / min by a Hanbon-NP7010C high-efficiency liquid phase pump. The speed is pumped into the heart-shaped static mixer B, and the temperature of the heat transfer oil in the heart-shaped static mixer B is set at 0°C, and the diazonium salt solution is obtained after the reaction. Set the temperature of the heat transfer oil in the heart-shaped tubular reactor to 80°C, and at the same time, use the Hanbon-NP7010C high-efficiency liquid phase pu...
Embodiment 3
[0036] At room temperature, the sulfuric acid solution with a mass percent concentration of 25% is pumped into the straight static mixer A with an electromagnetic metering pump at a speed of 49.9g / min, and then m-trifluoromethylaniline is pumped at a rate of 4.1g / min with an electromagnetic metering pump. The speed is pumped into the straight static mixer A, the temperature of the heat transfer oil in the straight static mixer A is set to 0°C, and the sodium nitrite solution with a mass percentage concentration of 30% is pumped at a rate of 6g / min by a Hanbon-NP7010C high-efficiency liquid phase pump. The speed is pumped into the heart-shaped static mixer B, and the temperature of the heat transfer oil in the heart-shaped static mixer B is set at 0°C, and the diazonium salt solution is obtained after the reaction. Set the temperature of the heat transfer oil in the heart-shaped tubular reactor to 100°C, and at the same time, use a Hanbon-NP7010C high-efficiency liquid phase pum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com