Use of imb-a6 as an androgen receptor antagonist
A technology of IMB-A6, 1. IMB-A6, applied in the field of new drug leads, can solve the problems of promoting the growth of prostate cancer cells and affecting the curative effect, and achieving the effect of contraceptive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1I
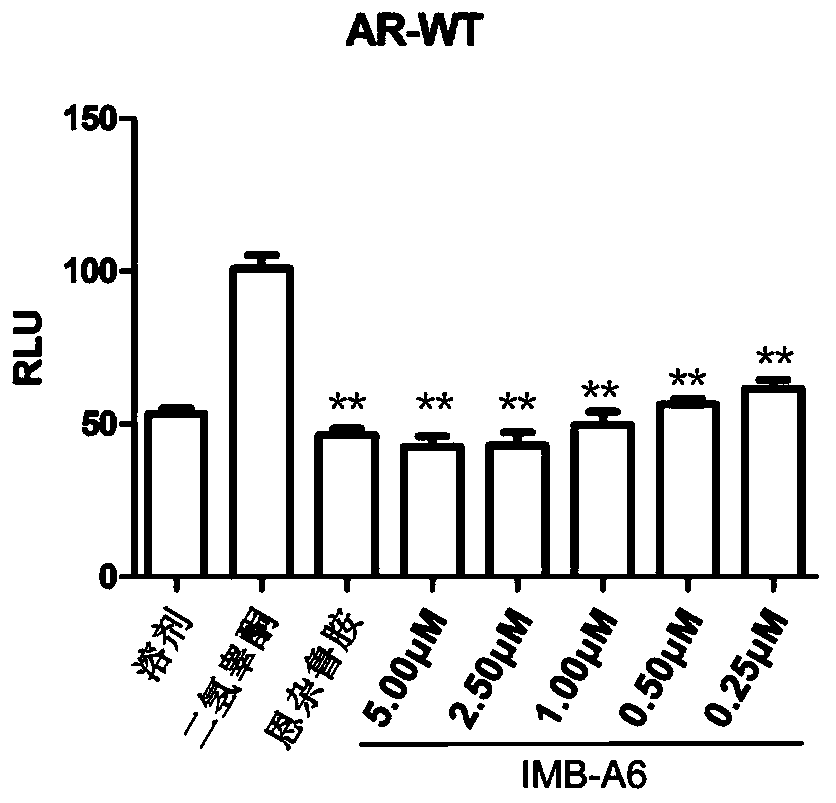
[0033] Embodiment 1IMB-A6 can suppress the activity of wild-type androgen receptor
[0034] will be 1.4×10 5PC-3 cell suspension per mL was inoculated in a 24-well plate at 500 μL per well. When the cells grew to 80%, each well was co-transfected with 100ng PSA-luc, 20ngAR-WT and 1ng pCMV-Renilla plasmids. 6 hours after transfection, the medium was replaced with phenol red-free RPMI 1640 medium containing 10% charcoal-stripped FBS; after 24 hours of transfection, one group was treated with 1 nM DHT + corresponding concentration of IMB-A6 or Enzalu in each well Add 1 μL of amines to each well, and add 1 μL of DMSO or corresponding concentration of IMB-A6 or enzalutamide to each well of the other group, and continue to culture for 24 hours. Finally, suck off the medium, add 100 μL of 1×PLB to each well to lyse for 20 minutes, collect the cell lysate into a clean EP tube, centrifuge, take 20 μL of the supernatant into a clean white 96-well plate, and follow the instructions of ...
Embodiment 2I
[0036] Example 2IMB-A6 can inhibit the activity of endogenous androgen receptor
[0037] There may be overexpression of androgen receptor due to exogenous expression, and false positive results may be caused by the plasmid transfection process. Therefore, we performed an experiment that IMB-A6 inhibits the activity of endogenous androgen receptor. The specific experimental steps refer to Example 1, the difference is that: LNCaP cells are used for this experiment, co-transfection is 100ng PSA-luc and 1ng pCMV-Renilla plasmid per well, and other operations are the same. Three parallel groups were set up in the experiment, and the statistical analysis was carried out, among which *P , and use GraphPad Prism 5.0 for graphing (see figure 2 ) and statistical analysis.
[0038] IMB-A6 had a dose-dependent inhibitory effect on the transcriptional activity of the endogenous AR of LNCaP, and this inhibitory effect was more pronounced in LNCaP cells. Comparing the results of LNCaP, i...
Embodiment 3I
[0041] Example 3 IMB-A6 can inhibit the activity of the mutant androgen receptor
[0042] At present, some series of AR mutations have been discovered, including tryptophan (W) → leucine (L) or cysteine (C) at position 741, threonine (T) → alanine at position 877 amino acid (A), 876 phenylalanine (F) → leucine (L). Among them, the W741C mutation is the main cause of bicalutamide resistance, and the T877A mutation is the main cause of hydroxyflutamide resistance. The specific experimental steps refer to Example 1, the difference is that AR-WT is replaced with AR-F876L or AR-T877A mutant plasmids during co-transfection, and other operations are the same. Three parallel groups were set up in the experiment, and statistical analysis was carried out, among which *P , and use GraphPad Prism 5.0 for graphing (see image 3 ) and statistical analysis.
[0043] The experimental results showed that IMB-A6 also had a good inhibitory effect on the transcriptional activity of F876L mut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com