Blood platelet antibody specificity identification method and kit thereof
A specific, platelet technology, applied in the field of medical testing, can solve the problems of inability to detect platelet antibodies, inaccurate test results, and complex testing process, and achieve the effects of accurate results, simple steps, and high efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Extraction of HLA Class I Glycoprotein Antigens
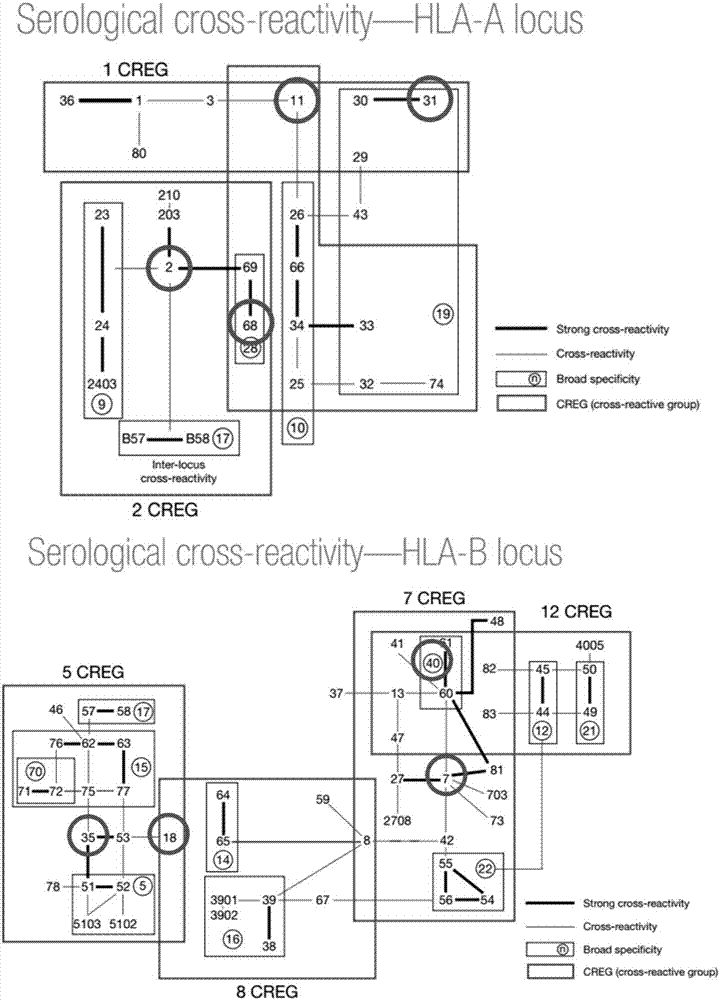
[0046] Investigate clinically meaningful HLA class Ⅰ antibody categories in my country, combined with the cross-reactivity characteristics of HLA class Ⅰ antigens, minimize the probability of missed detection of HLA class Ⅰ antibodies, and design the HLA class Ⅰ glycoprotein antigen combinations to be extracted as follows: A2, A11, A31, A68, B7, B 18, B35 and B40 antigens, HLA class Ⅰ glycoproteins cover the antigen map see figure 1 . Therefore, this embodiment provides a method for extracting the HLA class I glycoprotein antigen of the above antigen combination, and the extraction steps are as follows:
[0047] (1) Cultivate and collect human leukocytes or platelets expressing the above-mentioned relevant HLA class I glycoprotein antigens, store them at -80°C, and extract 3×10 9 cells;
[0048] (2) Take 3×10 9 Each of the human leukocytes or platelet cells was washed 3 times with normal saline, and the pack...
Embodiment 2
[0053] Example 2 Extraction of Platelet Glycoproteins GPⅡb / Ⅲa, GPⅠa / Ⅱa, GPⅠb / Ⅸ and GPⅣ
[0054] This embodiment provides a method for extracting platelet glycoproteins GPⅡb / Ⅲa, GPⅠa / Ⅱa, GPⅠb / IX and GPⅣ, comprising the following steps:
[0055] (1) Take fresh platelet-rich plasma (PRP) at 1300g, centrifuge for 10 minutes, discard the supernatant, and obtain the platelet-packed cells (5×10 10 ), washed 3 times with 0.1M pH 7.4 PBS (containing 2.5mM EDTA);
[0056] (2) Cleavage: Add 50 mL of lysate (the lysate is 10 mM Tris-HCl buffer at pH 7.4, containing 0.15 M of NaCl, 1 mM of Benzylbenzene) to the platelet packed cells after the above washing Sulfonyl fluoride, 2.5mM EDTA and 0.5% (v / v) Lubrol PX), cleavage at 4°C for 45 minutes, shake and mix well during the period, then add 50mL of the above-mentioned lysate, and lyse at 4°C for 30 minutes to make it Fully lysed, ready for use;
[0057] (3) After centrifuging at 100,000g for 30 minutes at 4°C, keep the supernatant (conta...
Embodiment 3
[0061] Example 3 Establishment of anti-human platelet monoclonal antibody hybridoma cell line and preparation and purification of monoclonal antibody
[0062] This embodiment provides the establishment of an anti-human platelet monoclonal antibody hybridoma cell line and the preparation and purification of the monoclonal antibody, including the following steps:
[0063] (1) Collect fresh type O whole blood, centrifuge at 200g for 10 minutes, extract the upper platelet-rich plasma, then centrifuge at 1000g for 10 minutes, discard the supernatant, keep the packed platelets, and wash with 0.01M pH 7.4 PBS buffer (containing 0.5% (w / v) EDTA) was washed twice, and the final platelet concentration was adjusted to 10 8 platelet suspension per mL; take 0.5 mL of the above platelet suspension to immunize mice through the abdominal cavity and subcutaneously on the back, for a total of 4 times with an interval of 10 days. Two days before the fusion, 0.2 mL of the above platelet suspensi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com