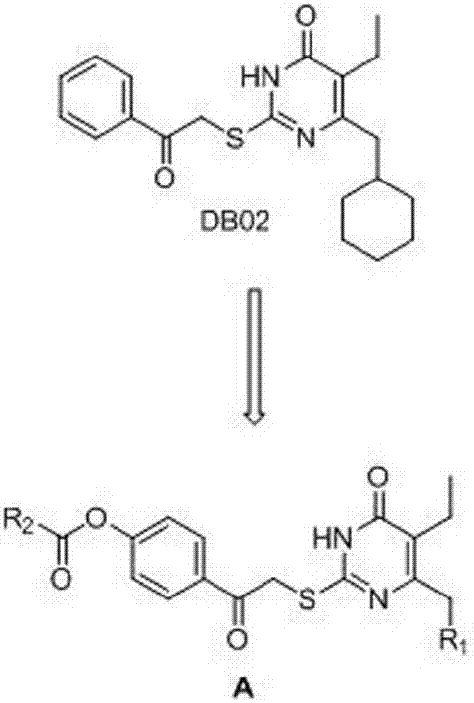
6-cyclohexylpyrimidone HIV reverse transcriptase inhibitor, preparation method and application thereof
A cyclohexylmethylpyrimidinone and reverse transcriptase inhibition technology, applied in the field of its preparation, 5-isopropyl-2--6-cyclohexylmethylpyrimidinone compounds, can solve drug resistance and side effects , HIV virus is easy to mutate and other problems, to achieve the effect of small cytotoxicity, high selectivity index, and significant anti-HIV-1 virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] Example 1: Synthesis of ethyl 4-cyclohexyl-2-isopropyl-3-oxobutanoate
[0024] Combine 52.4g activated zinc powder and 450ml anhydrous THF in N 2 Add a few drops of ethyl α-bromoisovalerate 2 dropwise under protection to initiate the reaction, reflux and stir at 66°C for 45 minutes until the reaction solution turns dark green, add 20.324g (0.165mol) cyclohexyl acetonitrile at one time, and within 1h Add 2 (88.71g, 0.4243mol) slowly, stop heating after continuing to reflux for 1H, cool to room temperature, add 80ml of 50% K 2 CO 3 The solution was vigorously stirred for 10 min, the reaction solution was allowed to stand and separated, the organic phase was separated, the residue was extracted with THF, the organic phase was combined, 150 ml of 15% HCl was added for hydrolysis, the organic layer was separated and concentrated under reduced pressure, and the aqueous layer was extracted with ethyl acetate for 3 times, the organic phases were combined, washed three times w...
example 2
[0025] Example 2: Preparation of 6-cyclohexyl-5-isopropyl-2-thiouracil
[0026] In a dry reaction flask, add 10g (0.43mol) of sodium metal in 300mL of absolute ethanol in batches, after the sodium is dissolved and cooled, add 24g (0.315mol) of thiourea at one time, and then dropwise add the above compound 3 (0.27mol ) in 20 mL of ethanol solution, heat the mixture to reflux for 5-7 hours, stop heating after TLC tracking until the compound 3 raw material point disappears, cool, evaporate the solvent under reduced pressure, dissolve the residue in 300 mL of water, adjust the pH value with concentrated hydrochloric acid It is about 6, a large amount of white precipitates are produced, filter with suction, wash the filter cake with water, and dry to obtain 6-cyclohexyl-5-isopropyl-2-thiouracil, which can be directly used for the synthesis of the target compound in the next step without purification .
example 3
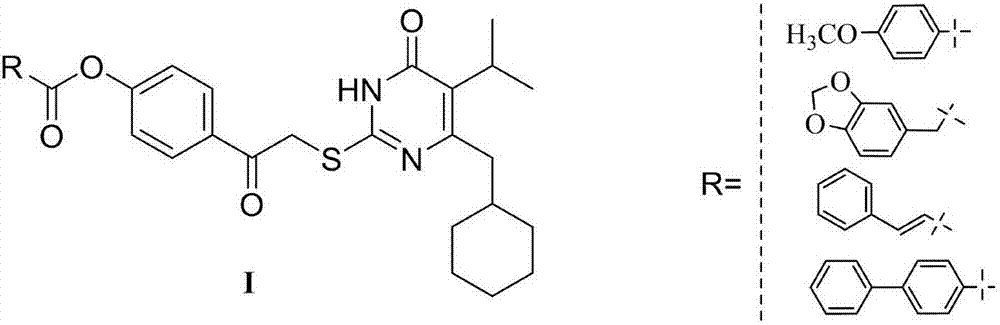
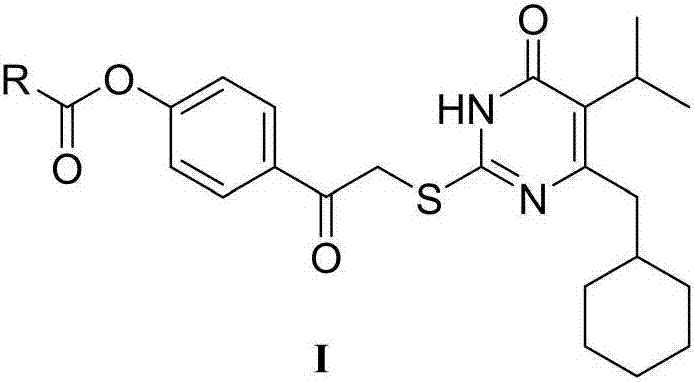
[0027] Example 3: Preparation of 5-isopropyl-2-(4-hydrocarbylformyloxyphenylcarbonylmethylthio)-6-cyclohexylpyrimidinone compound I
[0028] Dissolve 5-isopropyl-6-cyclohexylmethyl-2-thiouracil (2mmol) in 10ml of dry DMF solution, add K 2 CO 3 (2mmol), stirred and reacted at room temperature for half an hour, added 4-(2-bromoacetyl)phenyl ester 5 (2.2mmol), stirred and reacted at room temperature, TLC tracked until the raw material point disappeared, stopped the reaction, and poured the reaction solution into 50ml of ice water , stirring to precipitate a precipitate, filtering, washing the precipitate with water, suction filtration and drying to obtain a crude product, column chromatography or recrystallization to obtain a pure product.
[0029] Starting from 5-isopropyl-6-cyclohexyl-2-thiol-2,3-dihydropyrimidin-4(1H)-one, following the procedure described above, different 4-(2-bromoacetyl)benzene The ester undergoes alkylation reaction to obtain the I series compounds of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com