Preparation method of benzoic acid
A technology of benzoic acid and toluene, which is applied in the field of preparation of benzoic acid, can solve the problems of harsh reaction conditions and low selectivity of benzoic acid, and achieve the effects of mild conditions, good industrial application prospects, and reduced energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
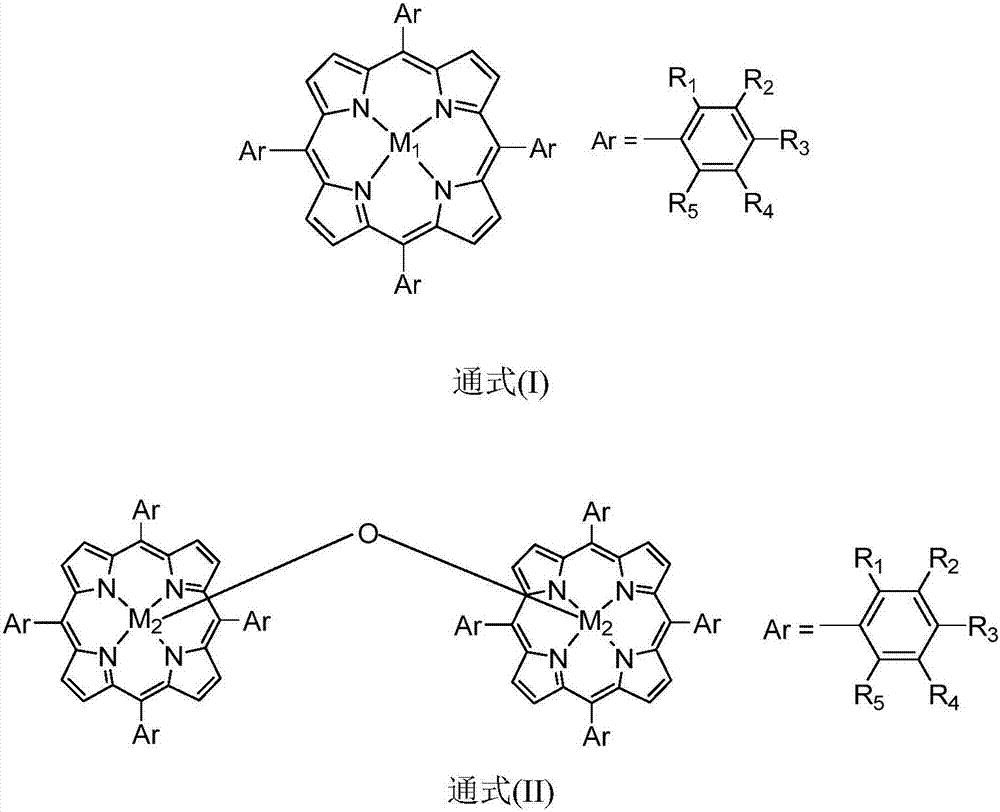
[0020] Contain 100ppm metalloporphyrin (M) with general formula (I) structure in 10mL 1 =Co,R 1 =Cl,R 2 = R 3 = R 4 = R 5 =H) in the acetonitrile solution, add 10mmol of toluene and 50mmol of diethyl malonate, and fill with 0.3MPa of oxygen, at a temperature of 130 ° C, stirring and reacting for 5 hours, through detection and analysis, the conversion rate of toluene is 30 %, the selectivity of benzoic acid is 86%.
Embodiment 2
[0022] Contain 1ppm metalloporphyrin (M) with general formula (I) structure in 10mL 1 =Mn,R 3 = NO 2 , R 1 = R 2 = R 4 = R 5 =H) in the 1,2-dichloromethane solution, add 10mmol of toluene and 5mmol of ethyl acetoacetate, and fill with 1.2MPa of oxygen, and stir the reaction at a temperature of 80°C for 5 hours. After detection and analysis, the toluene The conversion was 26% and the selectivity to benzoic acid was 92%.
Embodiment 3
[0024] Contain 10ppm metalloporphyrin (M) with general formula (I) structure in 10mL 1 =Cu,R 2 =CH 3 , R 1 = R 3 = R 4 = R 5 =H) in the DMF solution, add 10mmol of toluene and 10mmol of 2,4-pentanedione, and fill with 0.5MPa of oxygen, at a temperature of 100 ℃, stir the reaction for 5 hours, through detection and analysis, the conversion of toluene is 32%, the selectivity of benzoic acid is 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com