Method for recovering positive electrode material precursor and lithium carbonate from positive electrode waste material of lithium ion battery
A technology for lithium-ion batteries and positive electrode materials, which is applied in the field of efficient recovery of positive electrode material precursors and lithium carbonate, can solve the problems of comprehensive recovery of difficult and valuable metals, non-recyclable leaching agents, complex recovery processes, etc., to avoid secondary pollution , avoid impurity removal steps and extraction process, the effect of a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
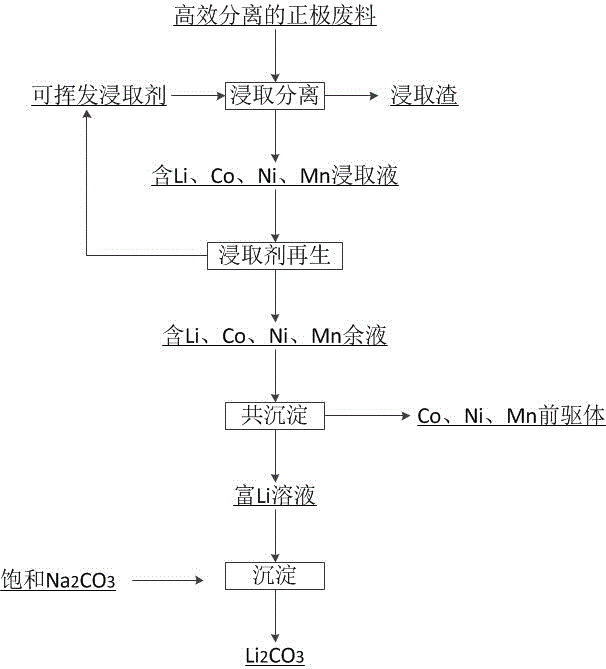
[0054] Lithium-ion battery positive electrode waste is leached with a mixed acid of nitric acid and citric acid containing a hydrogen peroxide reducing agent (the acid concentration is 2mol / L, and the mass percent content of the reducing agent is 3%). The leaching S / L is 50g / L, the leaching temperature is 40°C, the leaching time is 200min, and the stirring speed is 150rpm. The leaching solution and leaching residue containing Li, Ni, Co and Mn are obtained. The leaching rates of Li, Ni, Co and Mn were 92.21%, 90.60%, 91.20% and 92.5%, respectively, while the leaching rates of Al and Fe were only 1.32% and 2.76%. The leaching solution is subjected to high-temperature concentration / rectification treatment to recover the volatile leaching agent, and obtain a residual solution containing Co, Ni, Mn, and Li. The rectification / concentration temperature is 200° C., the time is 30 minutes, and the stirring speed is 150 rpm. The molar ratio of nickel, cobalt and manganese in the solu...
Embodiment 2
[0058] Lithium-ion battery positive electrode waste is leached with trichloroacetic acid and sulfuric acid mixed acid containing sodium sulfite reducing agent (acid concentration is 3mol / L, reducing agent mass percentage content is 4%). The leaching S / L is 50g / L, the leaching temperature is 40°C, the leaching time is 50min, and the stirring speed is 150rpm. The leaching solution and leaching residue containing Li, Ni, Co and Mn are obtained. The leaching rates of Li, Ni, Co and Mn were 94.60%, 92.80%, 93.20% and 93.80%, respectively, while the leaching rates of Al and Fe were only 2.12% and 3.46%. The leaching solution is subjected to high-temperature concentration / rectification treatment to recover the leaching agent, and obtain a residual solution containing Co, Ni, Mn, and Li. The molar ratio of nickel, cobalt and manganese in the solution conforms to the molecular formula LiNi x co y mn 1-x-y o 2 The molar ratio of Ni, Co and Mn in, where x>0, y>0, and x+y<1. Sodium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com