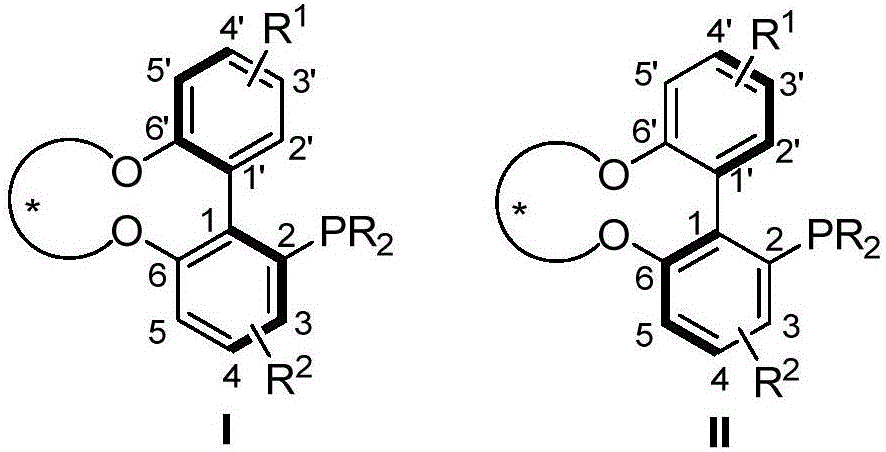
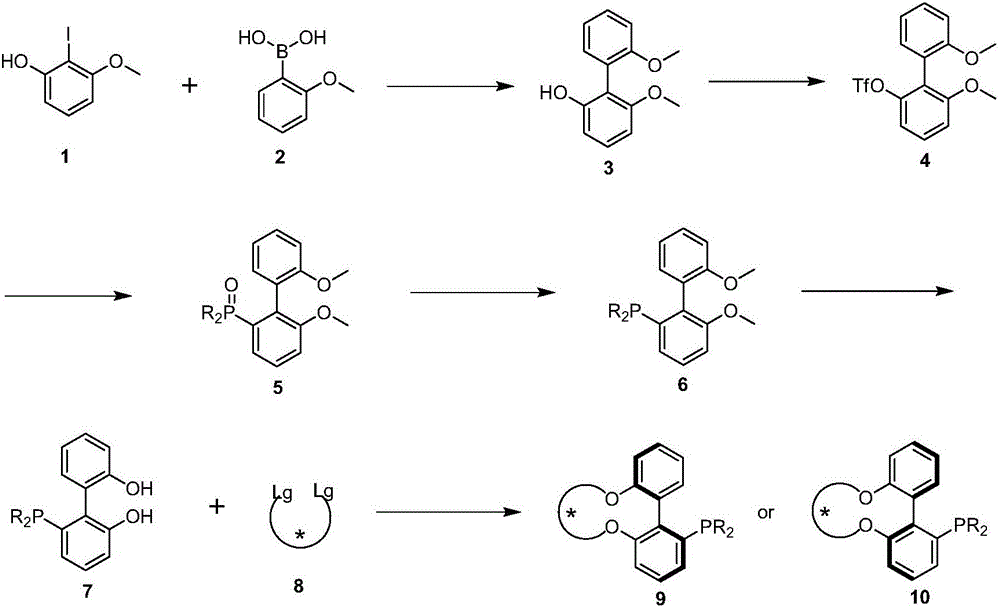
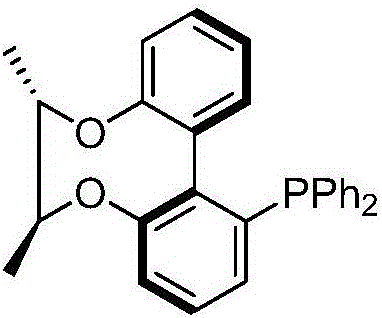
Axial chirality monophosphine ligand in chiral bridging and preparation method thereof
An axial chirality and chirality technology, which is applied in the field of chiral monophosphine ligands and their preparation, which can solve the problems of lack of ligands and catalytic systems, asymmetry, and limited catalytic examples.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Taking the preparation of (R)-[6,6'-((S,S)-2,3-butanedioloxy)]-2-diphenylphosphinebiphenyl as an example
[0031]
[0032] Preparation of 6,6'-dimethoxy-2-hydroxybiphenyl:
[0033] Under nitrogen protection, 15g (60mmol) 2-iodo-3-methoxyphenol 1, 13.68g (90mmol) 2-methoxyphenylboronic acid, 24.84g (180mmol) potassium carbonate, 538mg (2.4mmol) palladium acetate Suspend in 150mL N,N-dimethylformamide (DMF) and 150mL water, heat up to 50°C, stir for 24 hours, distill off DMF and water under reduced pressure, pour the reaction residue into 1mol / L hydrochloric acid, wash with acetic acid Ethyl ester was extracted 3 times, and the organic phases were combined. The organic phase was dried with anhydrous magnesium sulfate, and the crude product was purified by column chromatography to obtain 10.2 g of product 6,6'-dimethoxy-2-hydroxybiphenyl with a yield of 74%.
[0034] Product Analysis Results: 1 H NMR (400MHz, CDCl 3 ): δ7.41-7.45(m,1H),7.27-7.33(m,2H),7.0...
Embodiment 2
[0050] Example 2: Taking the preparation of (S)-[6,6'-((2R,4R)-2,4-pentanedioloxy)]-2-diphenylphosphinebiphenyl as an example
[0051]
[0052] Under nitrogen protection, 1.2g (3.24mmol) of 6,6'-dihydroxy-2-diphenylphosphinebiphenyl, 2.43g (7.45mmol) of cesium carbonate were suspended in 80mL of dry N,N-dimethylformaldehyde Amide (DMF), stirred at 80°C for 1 hour. Then, 2.67 g (6.48 mmol) of p-toluenesulfonate of (2S,4S)-2,4-pentanediol was dissolved in 40 mL of dry DMF, and slowly added dropwise to the reaction liquid over 4 hours. The reaction solution was continuously stirred at 80° C. for 30 hours, and DMF was distilled off under reduced pressure. The reaction residue was poured into 1mol / L hydrochloric acid, extracted three times with ethyl acetate, and the organic phases were combined. The organic phase was dried with anhydrous magnesium sulfate, and the crude product was purified by column chromatography to obtain 0.31 g of product (S)-[6,6'-((2R,4R)-2,4-pentanedio...
Embodiment 3
[0054] Example 3: Taking the preparation of (S)-[6,6'-((2R,5R)-2,5-hexanedioloxy)]-2-diphenylphosphine biphenyl as an example
[0055]
[0056]Under nitrogen protection, 1.2g (3.24mmol) of 6,6'-dihydroxy-2-diphenylphosphinebiphenyl, 2.43g (7.45mmol) of cesium carbonate were suspended in 80mL of dry N,N-dimethylformaldehyde Amide (DMF), stirred at 80°C for 1 hour. Then, 2.76 g (6.48 mmol) of p-toluenesulfonate of (2S,5S)-2,4-hexanediol was dissolved in 40 mL of dry DMF, and slowly added dropwise to the reaction liquid over 4 hours. The reaction solution was continuously stirred at 80° C. for 30 hours, and DMF was distilled off under reduced pressure. The reaction residue was poured into 1mol / L hydrochloric acid, extracted three times with ethyl acetate, and the organic phases were combined. The organic phase was dried with anhydrous magnesium sulfate, and the crude product was purified by column chromatography to obtain 0.61 g of the product (S)-[6,6'-((2R,5R)-2,5-hexanedi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com