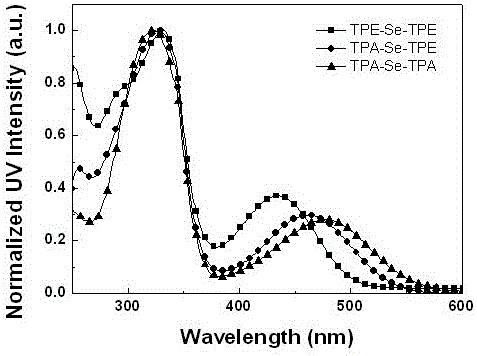
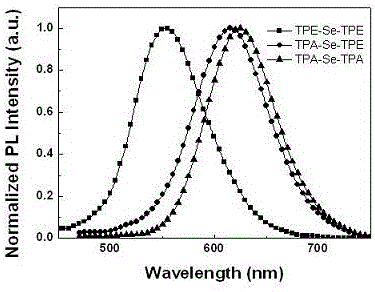
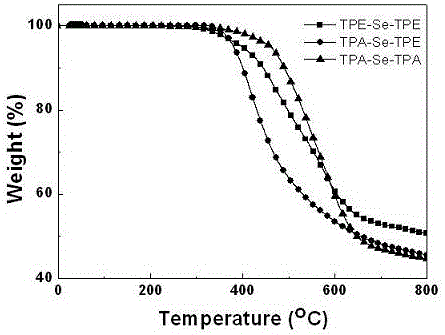
Preparation method and application of 2,1,3-benzoselenadiazole derivative
A technology of benzoselenodiazole and its derivatives, applied in the field of organic light-emitting materials, to achieve the effect of improving luminescent performance and avoiding quenching of aggregated states
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of 4,7-bis(1,2,2-triphenylethenyl)phenyl)-(2,1,3)-benzoselenodiazole(TPE-Se-TPE)
[0026]
[0027] 340mg (1.0mmol) 4,7-dibromo-(2,1,3)-benzoselenoadiazole, 1.145g (2.5mmol) 4-(1,2,2-tristyryl)-phenylboronic acid Pinacol ester, 10mL 2.0M potassium carbonate aqueous solution, and 50mL THF were added to a 100mL three-neck flask, and after 30 minutes of exhaust, 143mg Pd(PPh 3 ) 4 added to the reaction system. Stir and reflux for 24 hours to stop the reaction and cool to room temperature. The cooled reaction solution was poured into 40 mL of distilled water, extracted twice with DCM, and the obtained organic layer was washed with anhydrous MgSO 4 dry. The crude product was separated and purified by column chromatography (petroleum ether:dichloromethane=10:1) to obtain 565 mg of a yellow solid product (yield: 67%).1 H NMR (400MHz, CDCl 3 ,TMS)δ(ppm): 7.68~7.66(d, J=8.28Hz, 4H), 7.56(s, 2H), 7.19~7.16(d, J=8.32Hz, 4H), 7.13~7.09(m, 26H ),7.06~7.04(m,4H). 1...
Embodiment 2
[0030] 4-(N,N-diphenylamino)phenyl-7-(1,2,2-triphenylethenyl)phenyl-(2,1,3)-benzoselenadiazole (TPA-Se -TPE) synthesis
[0031]
[0032] (1) Synthesis of 4-bromo-7-(N,N-diphenylamino)phenyl-(2,1,3)-benzoselenadiazole
[0033] 1.12g (3.0mmol) 4,7-dibromo-(2,1,3)-benzoselenoadiazole, 1.11g (3.0mmol) 4-(diphenylamino) phenylboronic acid pinacol ester and 15mL Add 2.0M potassium carbonate aqueous solution and 70mL THF into a 100mL three-neck flask, exhaust for 0.5h, and 172mg Pd(PPh 3 ) 4 added to the reaction system. Stir and reflux for 24 hours to stop the reaction and cool to room temperature. The cooled reaction solution was poured into 60 mL of distilled water, extracted twice with DCM, and the obtained organic layer was washed with anhydrous MgSO 4 dry. The crude product was separated and purified by column chromatography (petroleum ether:dichloromethane=20:1) to obtain 894 mg of a yellow solid product (yield: 59%). 1 H NMR (400MHz, CDCl 3 ,TMS)δ(ppm): 7.96~7.94(d...
Embodiment 3
[0038] Synthesis of 4,7-bis(N,N-diphenylamino)phenyl)-(2,1,3)-benzoselenoadiazole(TPA-Se-TPA)
[0039]
[0040] 340mg (1.0mmol) 4,7-dibromo-(2,1,3)-benzoselenadiazole, 928mg (2.5mmol) 4-(diphenylamino) phenylboronic acid pinacol ester and 10mL 2.0M Potassium carbonate aqueous solution and 60mL THF were added to a 100mL three-necked flask, and exhausted for 30 minutes, 143mg Pd(PPh 3 ) 4 added to the reaction system. Stir and reflux for 24 hours to stop the reaction and cool to room temperature. The cooled reaction solution was poured into 50 mL of distilled water, and then extracted twice with DCM, and the obtained organic layer was washed with anhydrous MgSO 4 dry. The crude product was separated and purified by column chromatography (petroleum ether:dichloromethane=15:1) to obtain 509 mg of a yellow solid product (yield: 76%). 1 H NMR (400MHz, CDCl 3 ,TMS)δ(ppm): 7.80~7.78(d, J=8.64Hz, 4H), 7.59(s, 2H), 7.31~7.27(t, J=7.86Hz, 8H), 7.21~7.18(m, 12H ),7.07~7.04(t,J=7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum luminance | aaaaa | aaaaa |
| Maximum luminance | aaaaa | aaaaa |
| Maximum lumen efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com