Nitroethylene-containing ester compound as well as preparation method and application thereof
A technology of ester compounds and nitroethylene, applied in the field of fructose-1,6-bisphosphatase inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
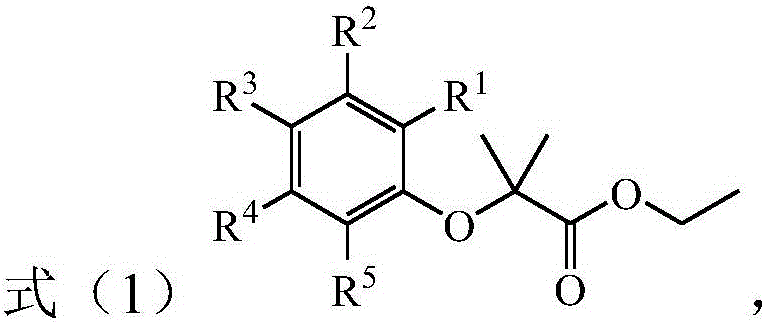
[0046] According to another preferred embodiment of the present invention, the ester compound is selected from the compounds described in the following formula:
[0047]
[0048] The present invention also provides a method for preparing the above-mentioned ester compound, the method comprising:
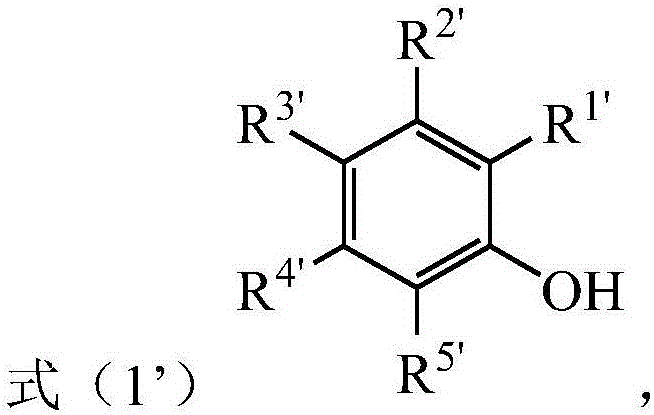
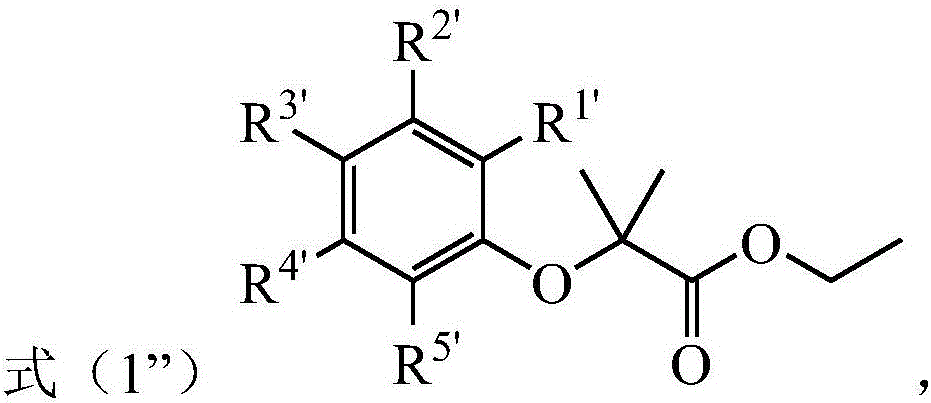
[0049] 1) In the presence of an alkali metal salt and a first organic solvent, the phenolic compound represented by the formula (1') is subjected to a substitution reaction with ethyl 2-halo-2-methylpropionate to obtain the formula (1") the indicated compound;
[0050]
[0051] Among them, R 1' -R 5' At least one of them is -CHO, and the remaining groups are independently selected from H, halogen, C1-C6 alkyl, C1-C6 alkoxy, -NO 2 , -COOH, -CN, -NR 2 , a C1-C6 alkyl group substituted by a substituent and a C1-C6 alkoxy group substituted by a substituent, R is selected from a C1-C6 alkyl group substituted or unsubstituted by a substituent, each of the substituents independen...
Embodiment 1
[0111] This example is used to illustrate the preparation of the ester compound represented by formula (1-1) of the present invention.
[0112] (1) Mix 0.701g (5mmol) of 4-fluoro-3-formylphenol, 10mmol of potassium carbonate and 25mL of acetonitrile, then add 1.5mL (about 10mmol) of ethyl 2-bromo-2-methylpropionate, and then Heat to reflux at 80°C for 6h; then cool the reaction solution to room temperature (about 25°C), filter with suction and wash the filter cake. The filtrate was collected, and the solvent of the filtrate was distilled off under reduced pressure. 20 mL of water and ethyl acetate (20 mL×3) were added for extraction, the organic phases were combined and washed with 20 mL of saturated brine, and the organic phase was dried over anhydrous sodium sulfate. Concentrate the dried organic phase, and purify the obtained concentrated solution by silica gel column chromatography (the eluent is a mixture of ethyl acetate and petroleum ether with a volume ratio of 1:4) t...
Embodiment 2
[0117] This example is used to illustrate the preparation of the ester compound represented by formula (1-2) of the present invention.
[0118] According to the method described in embodiment 1, the difference is,
[0119] In step (1), 4-chloro-3-formylphenol is used to replace 4-fluoro-3-formylphenol, thereby obtaining the compound (4.1mmol, yield rate 82%) shown in formula (1 "-2) ).
[0120] In step (2), then adopt the compound shown in formula (1 "-2) to replace the compound shown in formula (1 "-1), thereby make the ester compound (2.3mmol) shown in formula (1-2) , the yield was 77%).
[0121] 1 H NMR (600MHz, CDCl 3 )δ8.32(d, J=13.7Hz, 1H), 7.51(d, J=13.7Hz, 1H), 7.34(d, J=8.9Hz, 1H), 7.11(d, J=2.8Hz, 1H) ,6.90(dd,J=8.8,2.8Hz,1H),4.25(q,J=7.1Hz,2H),1.62(s,6H),1.26(t,J=7.1Hz,3H).
[0122] 13 C NMR (151MHz, CDCl 3 )δ173.44, 154.46, 138.85, 134.94, 131.01, 128.96, 128.83, 123.19, 118.89, 79.91, 61.75, 25.24, 14.08.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com