Metal organic frameworks (MOFs) material capable of photocatalyzing water cracking and preparation method thereof
A metal-organic framework, photocatalytic technology, applied in chemical instruments and methods, non-metallic elements, physical/chemical process catalysts, etc., can solve the problem of no discovery, and achieve the effect of simple method, universal applicability, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of Al-ATA with photocatalytic oxygen production activity
[0039] Dissolve 1.05mmol of aluminum nitrate in 20mL of N,N-dimethylformamide, then add 1.56mmol of 2-aminoterephthalic acid, stir thoroughly for 30 minutes, and transfer to a 100mL polytetrafluoroethylene reactor. Put the reactor into an oven at 110°C for 48 hours. Naturally cooled at room temperature, the reaction product was suction-filtered, the sample was washed three times with N,N-dimethylformamide and absolute ethanol, and dried at 60°C for 12 hours. The obtained light yellow powder was Al-ATA.
Embodiment 2
[0040] Embodiment 2: the preparation of Al-ATA-Ni
[0041] Disperse 100 mg of Al-ATA prepared in Example 1 in 100 mL of water, add 0.02 mmol of nickel nitrate, stir at room temperature for 96 hours, and fully wash the physically adsorbed Ni on the surface with water. 2+ , dried at room temperature, that is.
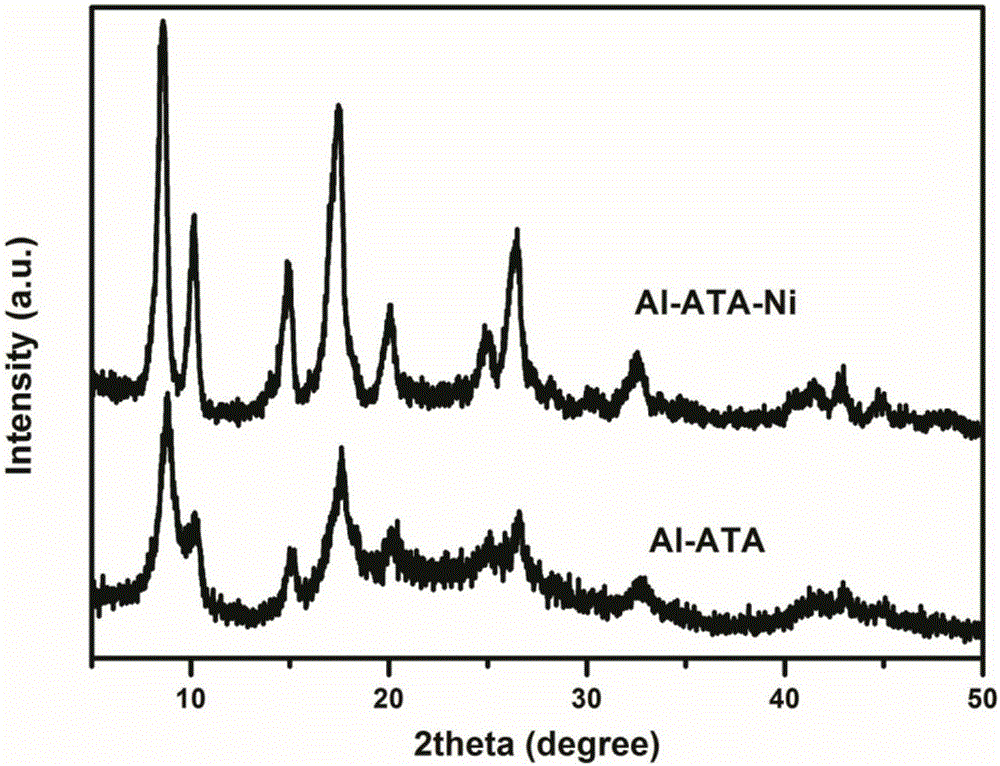
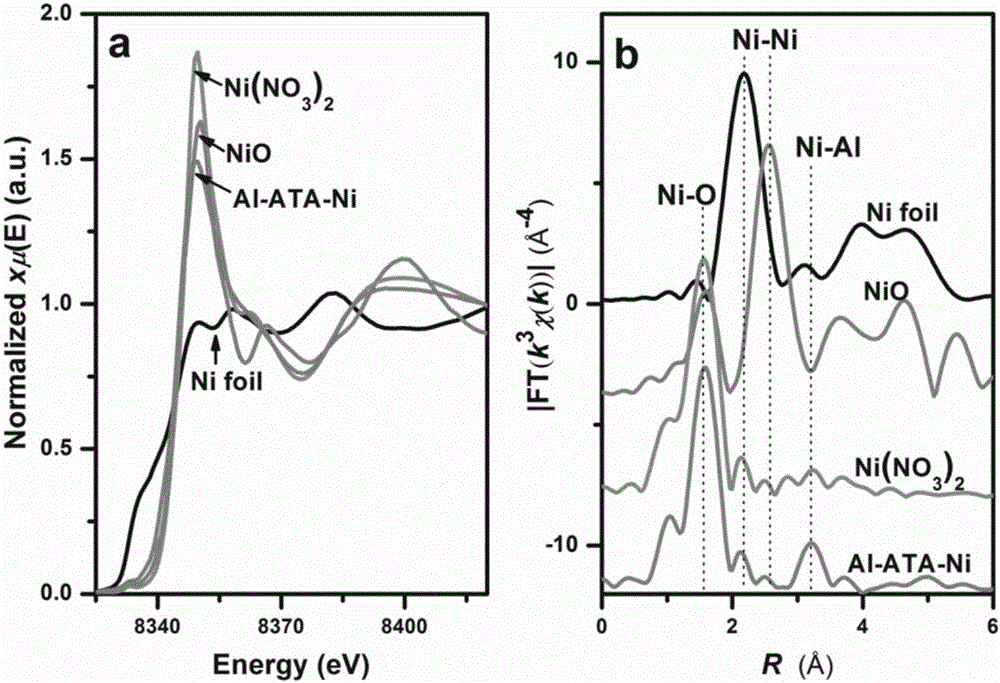
[0042] Carry out structural characterization to the product prepared in this embodiment, such as Figure 1-Figure 3 shown. in, figure 1 It is the X-ray diffraction figure of the product obtained in this embodiment 2, as can be seen from the figure, amino complex Ni in Al-ATA 2+ After that, the XRD of the material did not change significantly, indicating that the Ni 2+ The complexation did not destroy the crystal structure of the material Al-ATA. figure 2 It is the EXAFS figure of the product obtained in this embodiment, figure 2 The figure a in the middle shows that the coordination mode of Ni in Al-ATA-Ni is the same as that of nickel nitrate and NiO, and they ar...
Embodiment 3
[0043] Example 3: Activity test of Al-ATA-Ni photocatalytic splitting water
[0044] The experiment was carried out in a sealed glass system, and the side illuminated by the light source was quartz glass to ensure sufficient passage of ultraviolet light. The light source is a 300W xenon lamp. The reaction system is connected to a gas chromatograph to ensure in-situ online detection of hydrogen and oxygen generated in the system. Photocatalyst Al-ATA-Ni 30mg, deionized water 30mL. After the reaction system is sealed, first vacuumize for half an hour to remove the gas dissolved in the system, photocatalyst adsorption and solvent.
[0045] Figure 4 It is the rate diagram of the photocatalytic water splitting of the product made in this embodiment. It can be seen from the figure that the production rate ratio of hydrogen and oxygen is 2:1. It shows that the generated hydrogen and oxygen do come from the photocatalytic cracking of water.
[0046] In summary, the present inve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com