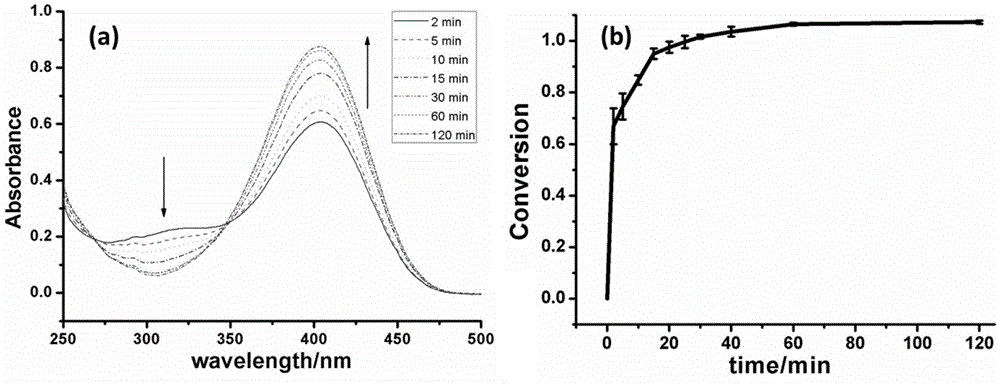
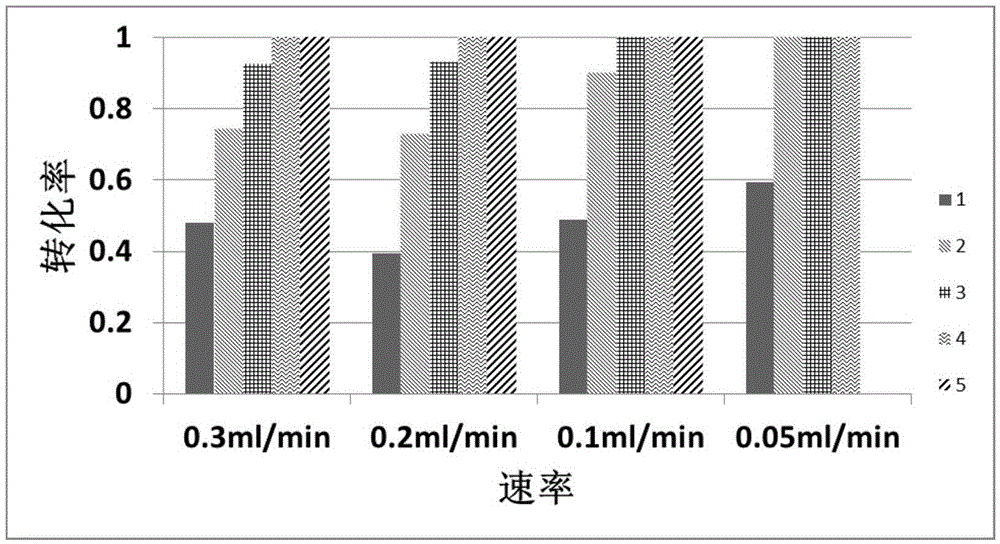
Method for catalytically hydrolyzing phosphatide bond of organic compound by virtue of cerium-based metal-organic frameworks
A technology of organic compounds and organic skeletons, applied in the field of catalytic hydrolysis, can solve problems such as practical application limitations and instability of natural hydrolytic enzymes, and achieve the effects of low preparation costs, low raw material prices, and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of cerium (III)-organic framework material: get 2.10g trimesic acid (H 3 BTC) was dissolved in 10mL N,N-dimethylformamide (DMF), and ultrasonically dissolved; take 4.34g cerium nitrate hexahydrate (Ce(NO 3 ) 3 ·6H 2 O) Dissolve in 45mL of water and ultrasonically dissolve; under magnetic stirring at 60°C, add the cerium nitrate solution dropwise to the trimesic acid solution, and it can be seen that the solution gradually becomes a white suspension. After continuing the reaction for 1 h, the reaction solution was centrifuged, washed with water, DMF, and ethanol several times in sequence to remove unreacted materials, and finally dried at 70°C. The resulting solid was a white powder.
Embodiment 2
[0034] Preparation of cerium (III)-organic framework material: Dissolve 2.10g of trimellitic acid in 10mL of N,N-dimethylformamide (DMF), and dissolve it by ultrasonic; take 4.34g of cerium nitrate hexahydrate (Ce( NO 3 ) 3 ·6H 2 O) dissolved in 45mL of water, ultrasonically dissolved; under magnetic stirring at 60°C, the cerium nitrate solution was added dropwise to the trimellitic acid solution. After continuing the reaction for 1 h, the reaction solution was centrifuged, washed with water, DMF, and ethanol several times in sequence to remove unreacted materials, and finally dried at 70°C.
Embodiment 3
[0036]Preparation of cerium (III)-organic framework material: Dissolve 1.66g of terephthalic acid in 10mL of N,N-dimethylformamide (DMF), and dissolve it by ultrasonic; take 4.34g of cerium nitrate hexahydrate (Ce( NO 3 ) 3 ·6H 2 O) dissolved in 45mL of water, ultrasonically dissolved; under magnetic stirring at 60°C, the cerium nitrate solution was added dropwise to the terephthalic acid solution. After continuing the reaction for 1 h, the reaction solution was centrifuged, washed with water, DMF, and ethanol several times in sequence to remove unreacted materials, and finally dried at 70°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com