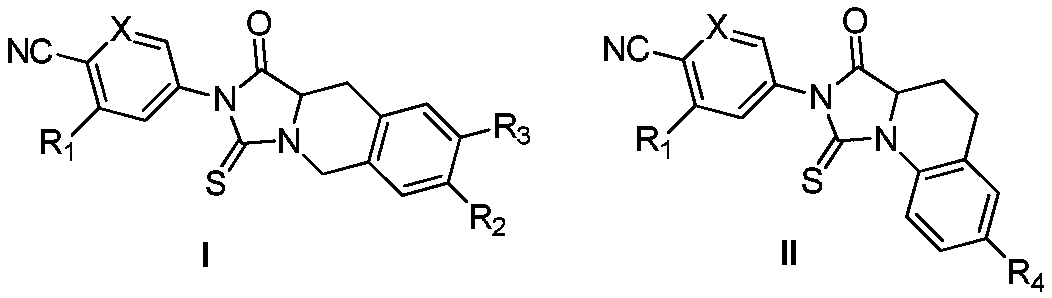
Thiohydantoin ternary and cyclic androgen receptor antagonists and uses thereof
A halogen and halogenated alkyl technology, applied in the field of medicinal chemistry, can solve problems such as ineffective treatment of CRPC, and achieve the effects of novel structure, high yield and easy availability of reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Synthesis of compounds of general formula I (CPU102-CPU113).
[0069]
[0070] Preparation of 4-cyano-3-fluorobenzyl bromide (2)
[0071] 3-Fluoro-4-cyanotoluene 1 (10g, 74.07mmol) was dissolved in 200mL of chloroform, 1g of BPO was added, stirred to dissolve, NBS (19.77g, 11.11mmol) was added in batches, refluxed for 12h, and the temperature was lowered. The sodium hydrogen aqueous solution was washed three times, an appropriate amount of water was washed three times, and an appropriate amount of saturated saline was washed three times, and the solvent was evaporated under reduced pressure. Column chromatography gave 14.00 g of a light yellow liquid with a yield of 88.79%. HRMS(ESI):m / z, calcd for C 8 h 5 BrFN 213.9678(M+H) + , found 213.9667.
[0072] Preparation of Diethyl 2-(3-fluoro-4-cyanobenzyl)-2-acetamidomalonate (3)
[0073] Add 100 mL of ethanol to a 250 mL three-necked flask, add sodium metal (0.76 g, 33.16 mmol) in batches, and stir at room temperat...
Embodiment 2
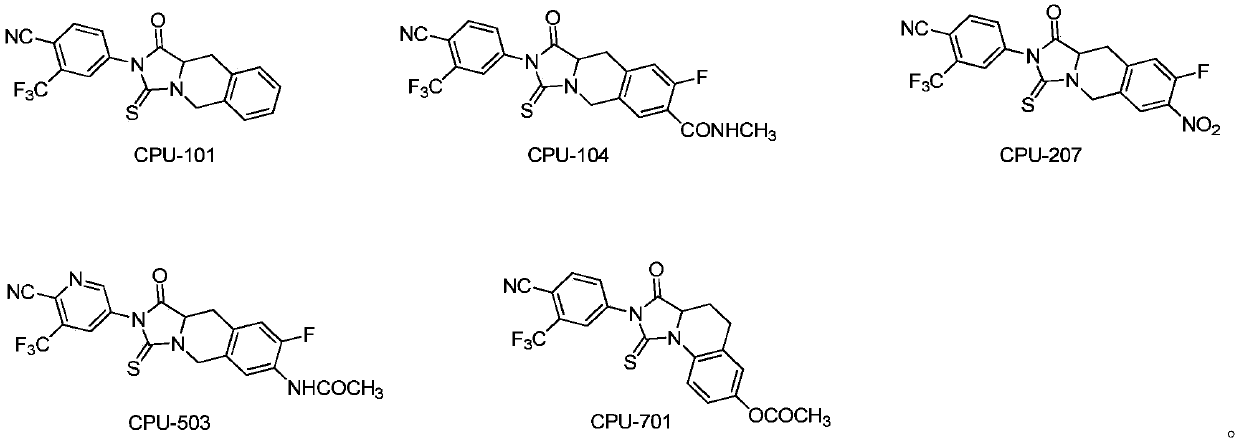
[0089] Preparation of Compound CPU103
[0090] Dissolve CPU102 (0.20g, 0.43mmol) in 5mL of tetrahydrofuran and 5mL of 1N aqueous sodium hydroxide solution, stir at room temperature for 50min, TLC detects that the reaction is complete, distill off the organic solvent under reduced pressure, adjust to pH = 6 with 1N HCl, precipitate a solid, and statically Set crystallization, suction filtration, column chromatography to obtain 0.15g yellow solid, yield 76.53%. m.p.160-162℃; 1 H-NMR (300MHz, DMSO-d 6 ):δ13.26(s,1H,-COOH),8.13(t,J=8.1Hz,1H,Ar-H),7.91(m,1H,Ar-H),7.71(d,J=8.1Hz, 1H,Ar-H),7.56(d,J=7.8Hz,1H,Ar-H),7.35(d,J=8.1Hz,1H,Ar-H),5.46(d,J=17.4Hz,1H, -CH-),4.75-4.84(m,2H,-CH 2 -),3.50-3.64(m,2H,-CH 2 -) ppm; 13 C-NMR (75MHz, DMSO-d 6 ):δ172.7,171.1,165.1,158.9,142.9,139.9,134.7,132.7,130.9,129.6,119.6,118.1,115.8,115.7,115.7,114.4,105.0,72.5,53.8,28.1ppm) HRMS( z, calcd for C 20 h 11 f 4 N 3 o 3 S 450.0456(M+H) + ,found 450.0530;IR(KBr):3413,2235,1718,1627,1443,13...
Embodiment 3
[0092] Preparation of Compound CPU104
[0093] Dissolve CPU103 (0.10g, 0.22mmol) in 10mL of dichloromethane, ice bath, add DMTMM (0.09g, 0.33mmol), stir for 30min, add triethylamine (0.09g, 0.88mmol), 30% methylamino alcohol Solution (0.03g, 0.33mmol), stirred at room temperature for 3h, TLC detected that the reaction was complete, evaporated the solvent to dryness under reduced pressure, added 1N HCl 20mL, extracted 3 times with 30mL ethyl acetate, combined the organic layer, divided 3 times with 30mL saturated sodium bicarbonate After washing, 30 mL of saturated brine was washed three times, the organic layer was dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was chromatographed to obtain 0.08 g of a yellow solid with a yield of 80.00%. m.p.329-333°C; 1 H-NMR (300MHz, DMSO-d 6 ): δ8.38(d, J=8.4Hz, 1H, Ar-H), 8.32(s, 1H, -NH-), 8.22(s, 1H, Ar-H), 8.02(d, J=8.4Hz ,1H,Ar-H),7.65(d,J=8.4Hz,1H,Ar-H),7.33(d,J=7.5Hz,1H,Ar-H),5.39(d,J=7.5Hz,1H ,-CH-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com