Pharmaceutical formulations for oral delivery of peptide or protein drugs
A protein and drug dosage form technology, applied in the fields of peptide/protein composition, drug combination, drug delivery, etc., can solve the problems that have not been raised
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0189] 1. A peptide or protein drug for use as a medicament having a molecular weight equal to or less than about 50 kDa, wherein the peptide or protein drug is orally administered in combination with:
[0190] A pharmaceutically acceptable copper salt / complex and / or a pharmaceutically acceptable zinc salt / complex; and
[0191] A pharmaceutically acceptable reducing agent.
[0192] 2. A pharmaceutically acceptable copper salt / complex for therapeutic use, wherein said copper salt / complex is administered orally in combination with:
[0193] a pharmaceutically acceptable reducing agent; and
[0194] Peptide or protein drugs having a molecular weight equal to or less than about 50 kDa.
[0195] 3. A pharmaceutically acceptable zinc salt / complex for therapeutic use, wherein said zinc salt / complex is administered orally in combination with:
[0196] a pharmaceutically acceptable reducing agent; and
[0197] Peptide or protein drugs having a molecular weight equal to or less than...
Embodiment 1
[0278] Example 1 : Compatibility of absorption enhancer with trace element copper
[0279] About them and the two main oxidation states of Cu 2+ and Cu + Compatibility Screening of Multiple Absorption Enhancers for the Trace Element Copper. The results of this test are shown in Table 1 below:
[0280]
[0281]
[0282] Table 1: Various absorption enhancers and Cu2 + and Cu + Salt Compatibility
[0283] The term clear solution as used in this table means that no significant visible precipitation or flocculation was observed. The term clear solution also includes slightly colored clear solutions such as yellowish or orange solutions.
[0284] Conclusions: Certain absorption enhancers such as medium chain fatty acid salts and derivatives are not well compatible with copper(II) salts. However, zwitterionic as well as nonionic surfactants show good compatibility with copper salts. In addition, monovalent copper also shows compatibility with sodium caprate and sodium...
Embodiment 2
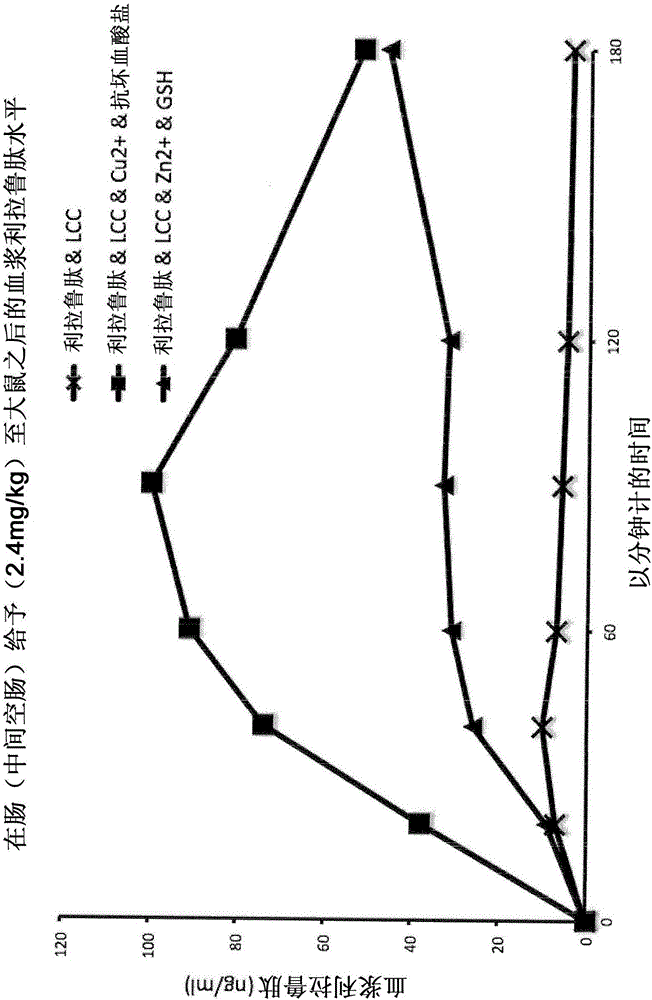
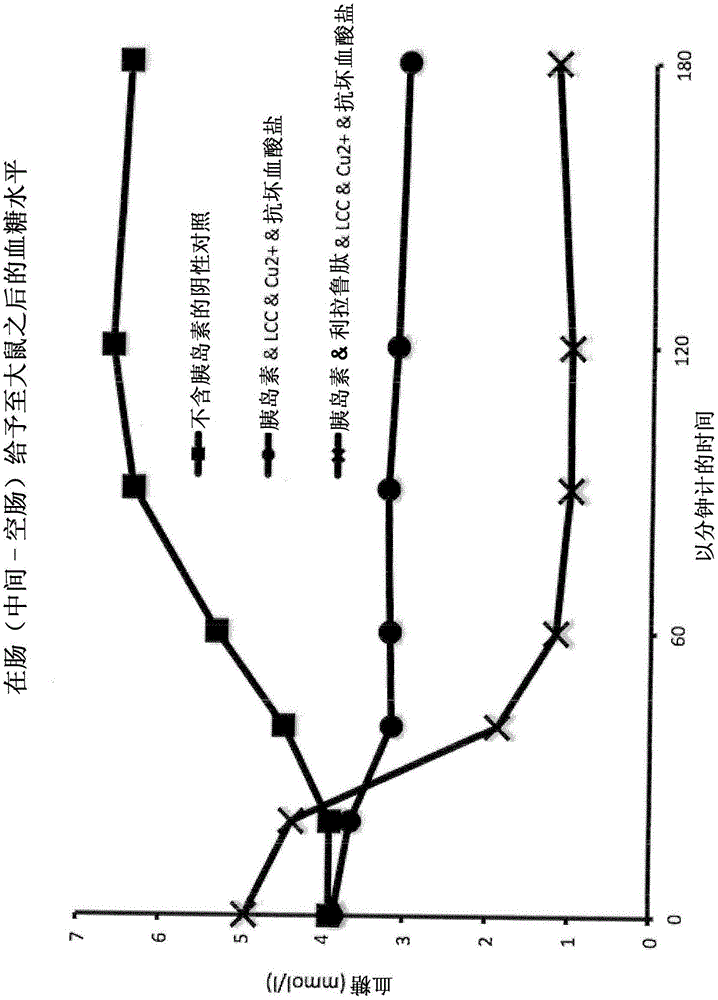
[0285] Example 2 : Pharmacodynamic and Pharmacokinetic Profiles of Human Insulin Preparations After Administration to the Middle Jejunum of Sprague Dawley Rats
[0286] Method - Administration to Rat Jejunum: Direct application of peptide or protein drugs and their formulations in the mid-jejunum (middle part of the small intestine - the jejunum) enables the study of their passage through the intestinal barrier and resistance to degradation by digestive enzymes Ability. Sprague-Dawley rats weighing 250-300 g were used. The study was performed under anesthesia. The results obtained are shown in Table 2 below:
[0287]
[0288] Table 2: Pharmacokinetic profiles of human insulin preparations
[0289] Conclusions: A simple physical blend of human insulin and the typical absorption enhancer sodium caprate in the presence of the trace element copper and ascorbate as a reducing agent moderately improves the oral bioavailability of human insulin. No more significant improveme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com