miRNA-155 and application of its inhibitor in DC-CIK cell culture
A technology of mirna-155, 1. mirna-155, applied in the field of immunization, can solve the problems of unsatisfactory effect and poor efficacy of adoptive immunization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
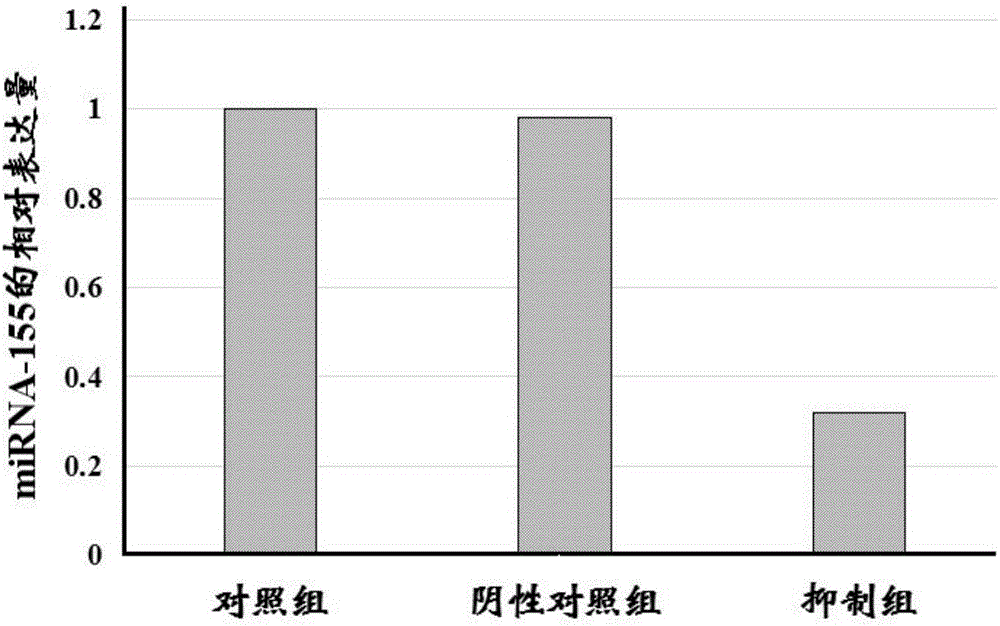
[0024] Example 1: The effect of miRNA-155 on DC-CIK cell co-culture
[0025] 1. Experimental materials
[0026] 1. miRNA-155 inhibitor is provided by Shanghai Jima Pharmaceutical Technology Co., Ltd. (sequence 1 below); inhibitor negative control is provided by Shanghai Jima Pharmaceutical Technology Co., Ltd. (sequence 2 below).
[0027] Sequence 1: 5’-ACCCCUAUCACGAUUAGCAUUAA-3’;
[0028] Sequence 2: 5’-CAGUACUUUUGUGUAGUACAA-3’.
[0029] 2. The tumor cells are leukemia cells, including K562 / A02, THP-1 and HL-60 cells.
[0030] 2. Experimental method
[0031] 1. The separation and culture of effector cells DC and CIK
[0032] (1) Separation of mononuclear cells: Collect 20mL of peripheral blood from healthy volunteers, dilute 1:1 with pre-cooled PBS, slowly add the upper layer of lymphocyte separation solution, 650g, centrifuge for 20min at 4°C, collect white cell layer, and separate mononuclear cells , Resuspend the cells in 1640 medium and place them at 37℃, 5% CO 2 Incubate for 2h in t...
Embodiment 2
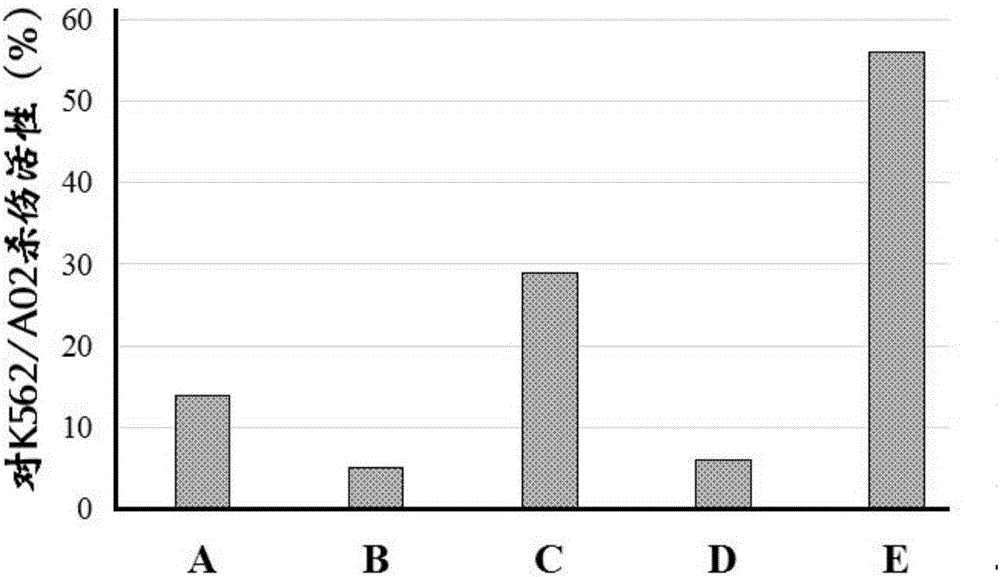
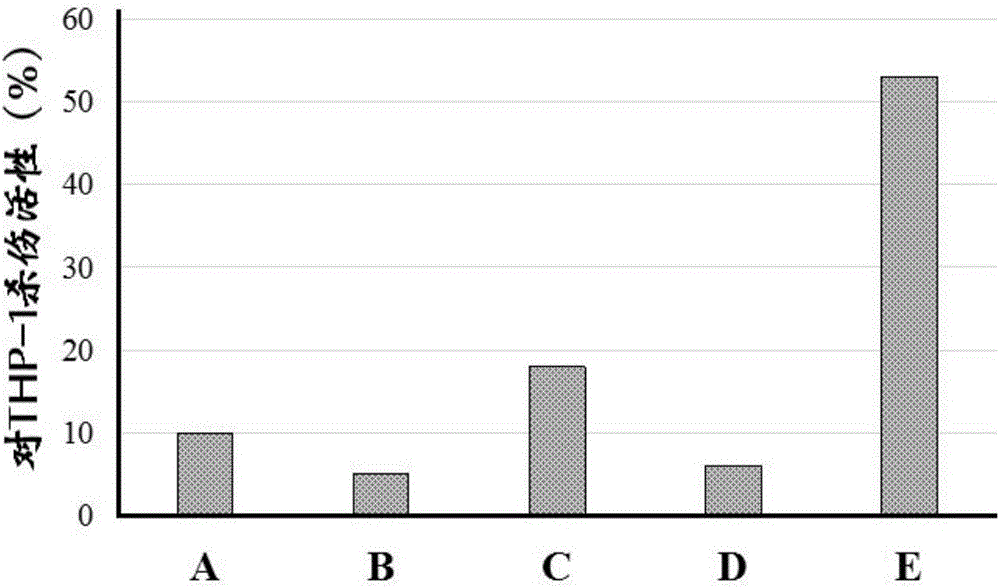
[0082] Example 2: Screening of miRNA-155 inhibitors (Pseudostellaria japonicus B, C)
[0083] 1. Experimental materials
[0084] Pseudoginsenopsis cyclic peptide B was purchased from Shanghai Yuanye Biotechnology Co., Ltd.; Pseudoginsenopsis cyclic peptide C was purchased from Kunming Institute of Botany, Chinese Academy of Sciences. Other materials are the same as in Example 1.
[0085] 2. Experimental method
[0086] 1. The separation and culture of effector cells DC and CIK
[0087] (1) Separation of mononuclear cells: Collect 20mL of peripheral blood from healthy volunteers, dilute 1:1 with pre-cooled PBS, slowly add the upper layer of lymphocyte separation solution, 650g, centrifuge for 20min at 4°C, collect white cell layer, and separate mononuclear cells , Resuspend the cells in 1640 medium and place them at 37℃, 5% CO 2 Incubate for 2h in the incubator.
[0088] (2) Cultivation of DC cells: After mononuclear cells are cultured for 2 hours, adherent cells are collected and induc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com