Preparation method for ravuconazole intermediate
A technology for rifaconazole and intermediates, applied in the field of preparation of rifaconazole intermediates, can solve the problems of low chiral purity, reduced chiral purity, long reaction steps, etc., and achieve high chiral purity and high yield High, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
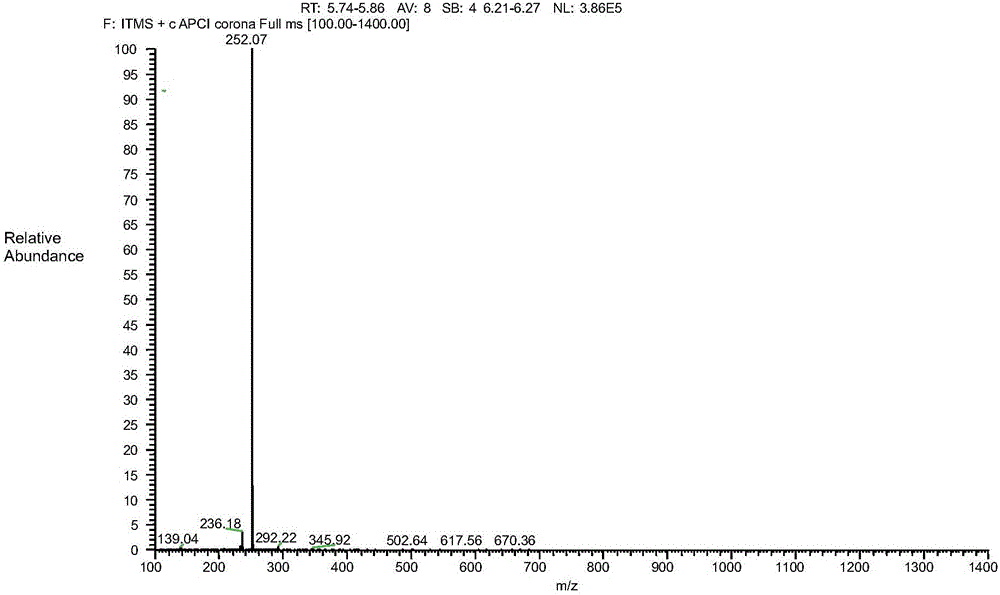
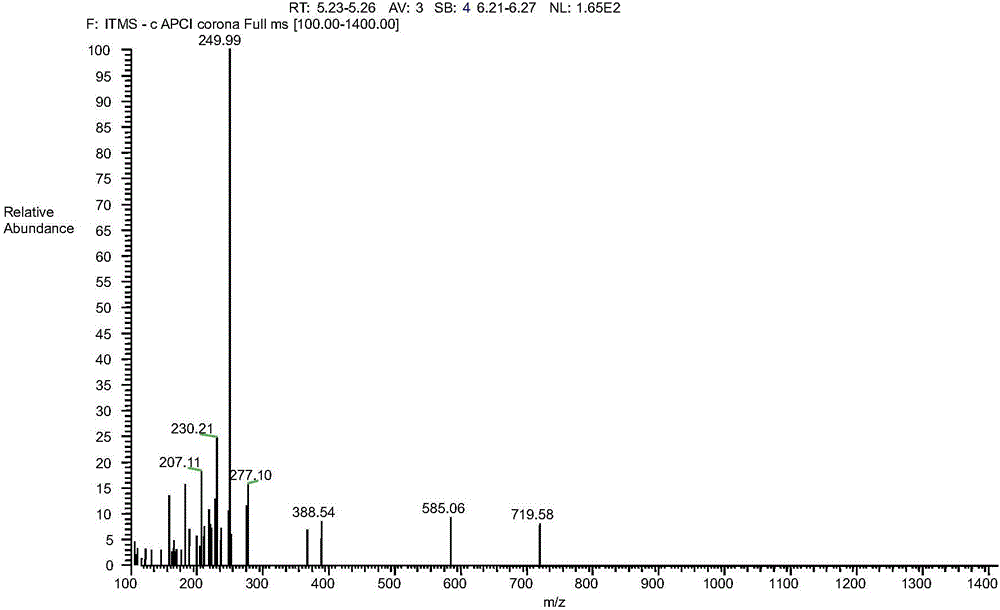
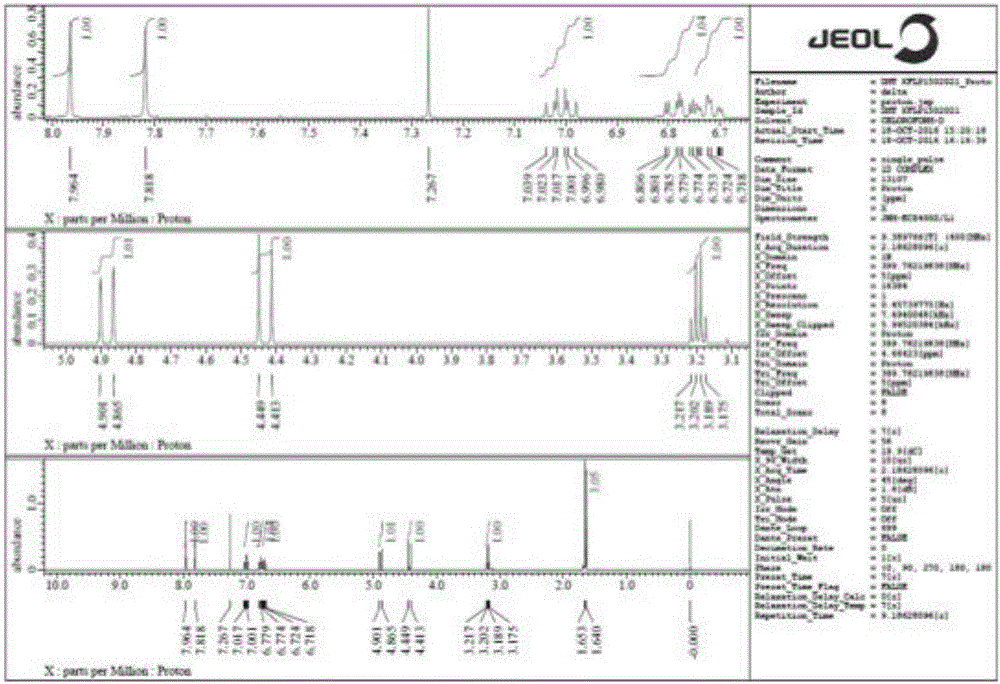
Image
Examples
Embodiment 1
[0035] Add 100g THF, 100g (0.96mol) methyl lactate, 12g (48mmol) pyridinium p-toluenesulfonate into a 1L three-necked flask, and drop in 86.2g (1.0mol) morpholine. =10, temperature controlled at 10°C and stirred for 3 hours, after the heat preservation and stirring, take a sample for GC (methyl lactate <1%), cool the system down to 5°C, continue to drop 84g (1mol) of 3,4-dihydropyran, drop Stir at 10°C for 15 hours after completion of temperature control (GC detection of intermediates <3%). After incubation, add 300 g of dichloromethane to the reaction solution, and wash the reaction solution once with 200 g of dilute hydrochloric acid with a mass fraction of 5%. Wash once with 5% sodium bicarbonate solution, separate the layers, add anhydrous sodium sulfate and molecular sieves to the organic phase and dry until the water content is less than 1000ppm. The solvent was removed to obtain 221.6 g of light yellow oil compound I, with a yield of 95% and a GC purity of 98%, and 200 ...
Embodiment 2
[0040]Add 100g THF, 100g (0.96mol) methyl lactate, 12g (48mmol) pyridinium p-toluenesulfonate into a 1L three-necked flask, and drop in 86.2g (1.0mol) morpholine. =10, temperature controlled at 10°C and stirred for 3 hours, after the heat preservation and stirring, take a sample for GC (methyl lactate <1%), cool the system down to 5°C, continue to drop 84g (1mol) of 3,4-dihydropyran, drop Stir at 10°C for 15 hours after completion of temperature control (GC detection of intermediates <3%). After incubation, add 300 g of dichloromethane to the reaction solution, and wash the reaction solution once with 200 g of dilute hydrochloric acid with a mass fraction of 5%. Wash once with 5% sodium bicarbonate solution, separate the layers, add anhydrous sodium sulfate and molecular sieves to the organic phase and dry until the water content is less than 1000ppm. The solvent was removed to obtain 221.6 g of light yellow oil compound I, with a yield of 95% and a GC purity of 98%, and 200 g...
Embodiment 3
[0044] Add 100g THF, 100g (0.96mol) methyl lactate, 12g (48mmol) pyridinium p-toluenesulfonate into a 1L three-necked flask, and drop in 86.2g (1.0mol) morpholine. =10, temperature controlled at 10°C and stirred for 3 hours, after the heat preservation and stirring, take a sample for GC (methyl lactate <1%), cool the system down to 5°C, continue to drop 84g (1mol) of 3,4-dihydropyran, drop Stir at 10°C for 15 hours after completion of temperature control (GC detection of intermediates <3%). After incubation, add 300 g of dichloromethane to the reaction solution, and wash the reaction solution once with 200 g of dilute hydrochloric acid with a mass fraction of 5%. Wash once with 5% sodium bicarbonate solution, separate the layers, add anhydrous sodium sulfate and molecular sieves to the organic phase and dry until the water content is less than 1000ppm. The solvent was removed to obtain 221.6 g of light yellow oil compound I, with a yield of 95% and a GC purity of 98%, and 200 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com