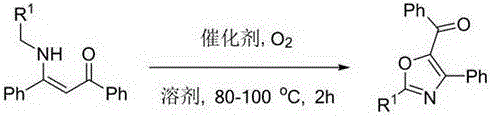
Novel method for synthesizing oxazole compound
A synthesis method and technology for synthesizing oxazole, applied in the direction of organic chemistry and the like, can solve the problems of long reaction time, low yield, expensive reaction raw material catalyst, etc., and achieve the effects of simple operation, good yield and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of (2,4-diphenyl-5-oxazolyl)phenylphenone:
[0025] Add 30% (6.7 mg) copper bromide, 0.1 mmol (31.3 mg) 3-(benzylamino)-1,3-diphenylprop-2-en-1-one in 3 mL DMF solution to the flask, in oxygen React at 80°C for 2 h in the atmosphere; after the raw materials are consumed, cool to room temperature, remove the solvent by suction filtration under reduced pressure, and then purify by silica gel column chromatography (ethyl acetate:petroleum ether=1:100) to obtain light yellow Solid compound 4a 23.48 mg, yield rate 75%, its structural formula is, ;
[0026] 1 H NMR (400 MHz, CDCl 3 ) δ 8.13 – 8.10 (m, 2H), 8.02 (ddd, J = 5.4, 2.9, 1.5 Hz, 2H), 7.89 (dd, J = 8.4, 1.3 Hz, 2H), 7.52 (d, J = 7.4 Hz, 1H), 7.48 –7.44 (m, 3H), 7.42 – 7.39 (m, 2H), 7.35 (dd, J = 5.1, 1.8 Hz, 3H). 13 C NMR (100 MHz, CDCl 3 ) δ 183.19, 161.96, 148.84, 143.50, 137.58, 132.87, 131.75, 130.63, 129.84, 129.58, 129.32, 129.00, 128.36, 128.22, 127.435, 126.
Embodiment 2
[0028] Synthesis of (2-(4-methoxyphenyl)-4-phenyl-5-oxazolyl)phenylmethanone:
[0029] Add 30% (6.7 mg) copper bromide, 0.1 mmol (34.3 mg) 3-(3-methoxybenzyl)amino)-1,3-diphenylprop-2-en-1-one to the flask 3 mL of DMF solution was reacted at 80 °C for 2 h in an oxygen atmosphere; after the raw materials were consumed, cooled to room temperature, the solvent was removed by suction filtration under reduced pressure, and then purified by silica gel column chromatography (ethyl acetate:petroleum ether=1 : 100) to obtain 28.15 mg of orange solid compound 4b with a yield of 82%, and its structural formula is: ;
[0030] 1 H NMR (400 MHz, CDCl 3 ) δ 8.08 (dd, J = 6.5, 3.0 Hz, 2H), 7.95 (d, J =7.3 Hz, 2H), 7.74 (d, J = 7.7 Hz, 1H), 7.70 (d, J = 2.1 Hz, 1H), 7.55 (d, J =7.4 Hz, 1H), 7.46 (d, J = 7.8 Hz, 2H), 7.41 (ddd, J = 13.9, 8.5, 5.5 Hz, 5H),3.87 (s, 3H). 13 C NMR (100 MHz, CDCl 3 ) δ 183.09, 161.81, 159.99, 148.81, 143.52, 137.58, 132.95, 130.16, 129.90, 129.65...
Embodiment 3
[0032] Synthesis of (2-(4-fluorophenyl)-4-phenyl-5-oxazolyl)phenylmethanone:
[0033] Add 30% (4.1 mg) copper chloride, 0.1 mmol (33.1 mg) 3-((4-fluorobenzyl)amino)-1,3-diphenylprop-2-en-1-one in 3 mL of DMF solution was reacted at 80°C for 2 h in an oxygen atmosphere; after the raw materials were consumed, cooled to room temperature, the solvent was removed by suction filtration under reduced pressure, and then purified by silica gel column chromatography (ethyl acetate:petroleum ether=1 : 100) to obtain 22.28 mg of orange solid compound 4c with a yield of 67.3%, and its structural formula is: ;
[0034] 1 H NMR (400 MHz, CDCl 3 ) δ 8.21 – 8.15 (m, 2H), 8.08 – 8.03 (m, 2H), 7.95 – 7.91 (m, 2H), 7.61 – 7.55 (m, 1H), 7.46 (t, J = 7.7 Hz, 2H), 7.41 (dd, J =4.1, 2.5 Hz, 3H), 7.23 – 7.15 (m, 2H). 13 CNMR (400 MHz, CDCl 3 )δ 183.18, 166.20,163.68, 161.13, 148.81, 143.55, 137.54, 134.89, 132.93, 130.54, 129.91,129.80, 129.71, 129.56, 129.32, 129.04, 128.39, 128.26, 122.75,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com