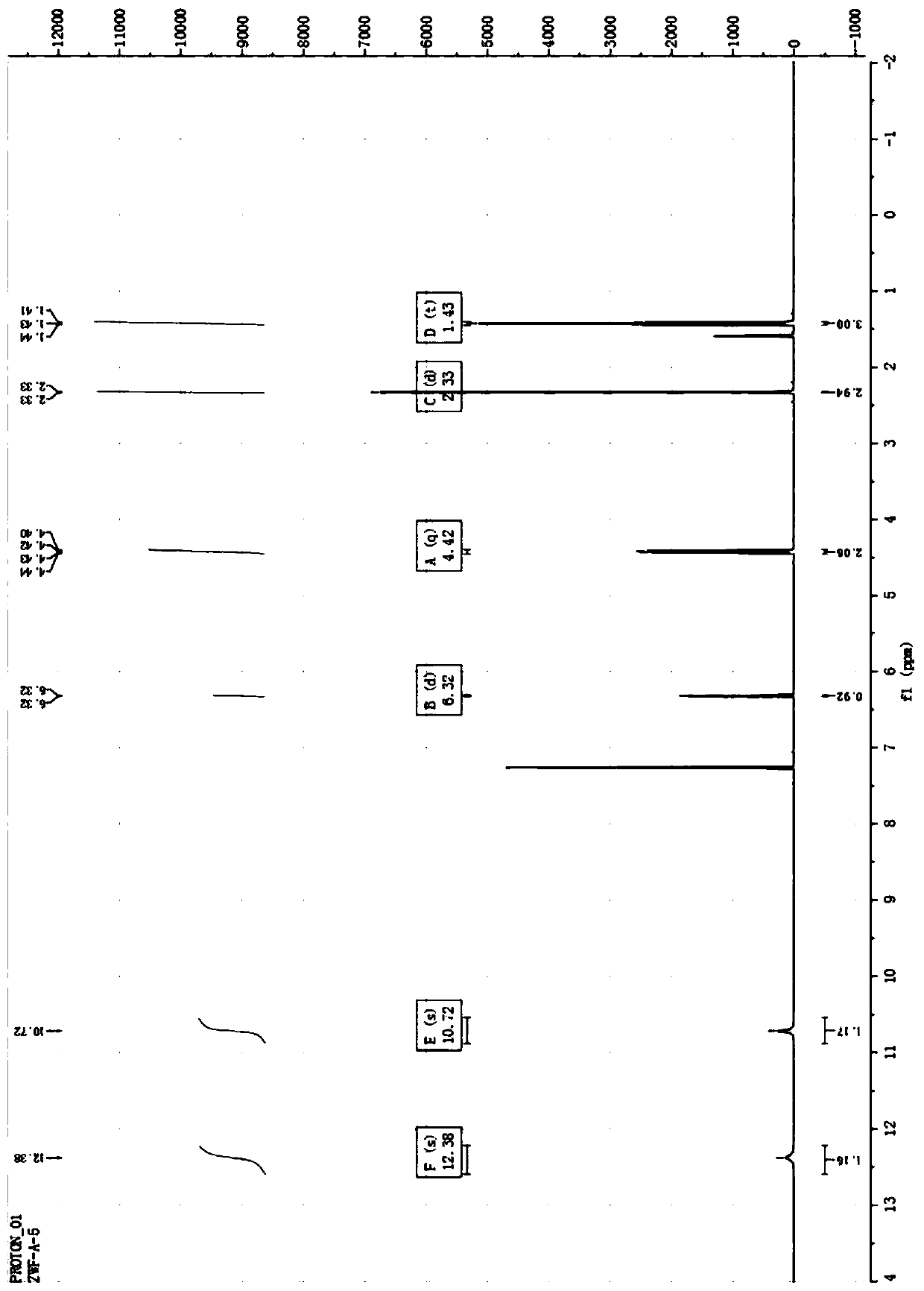
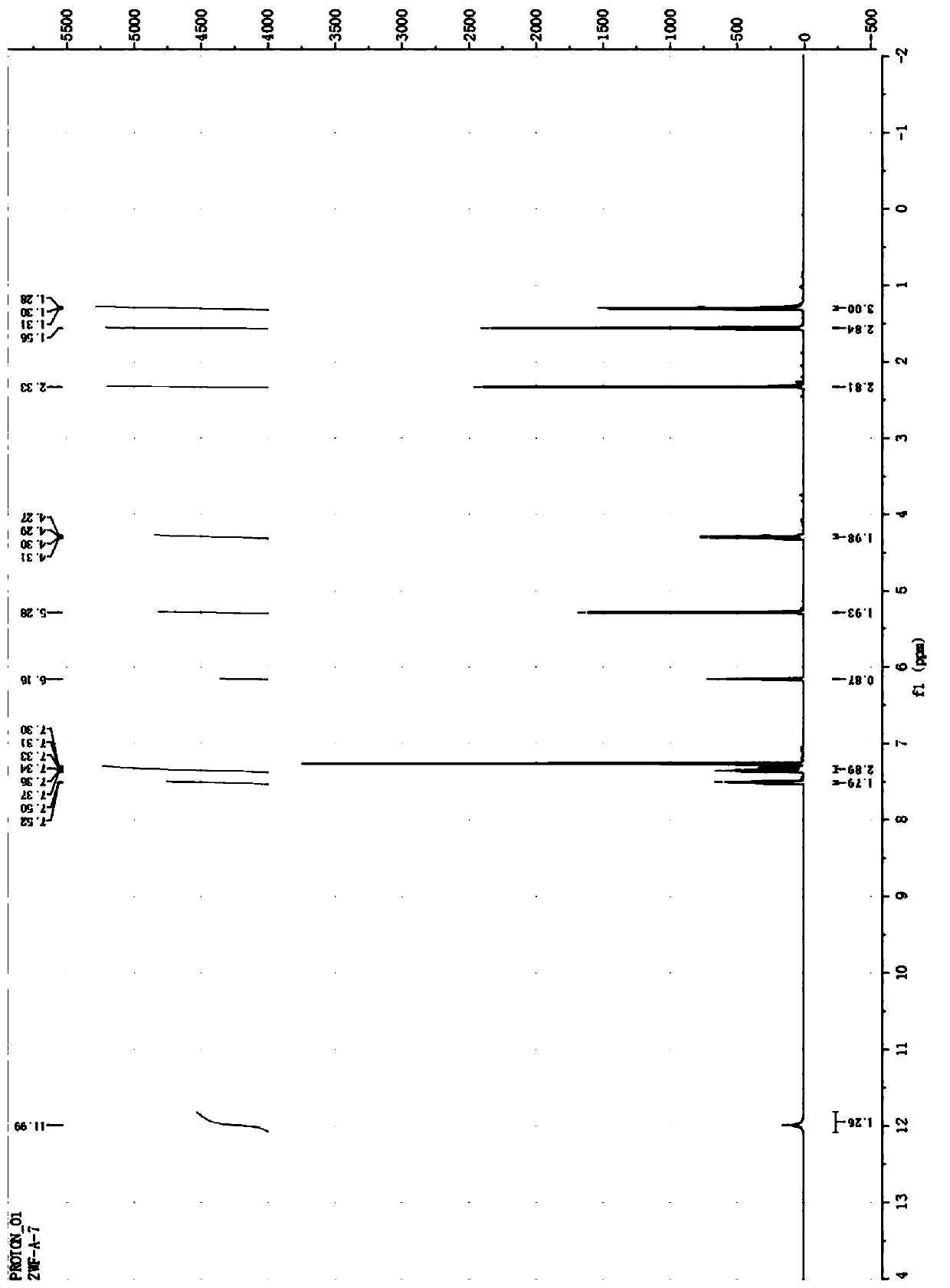
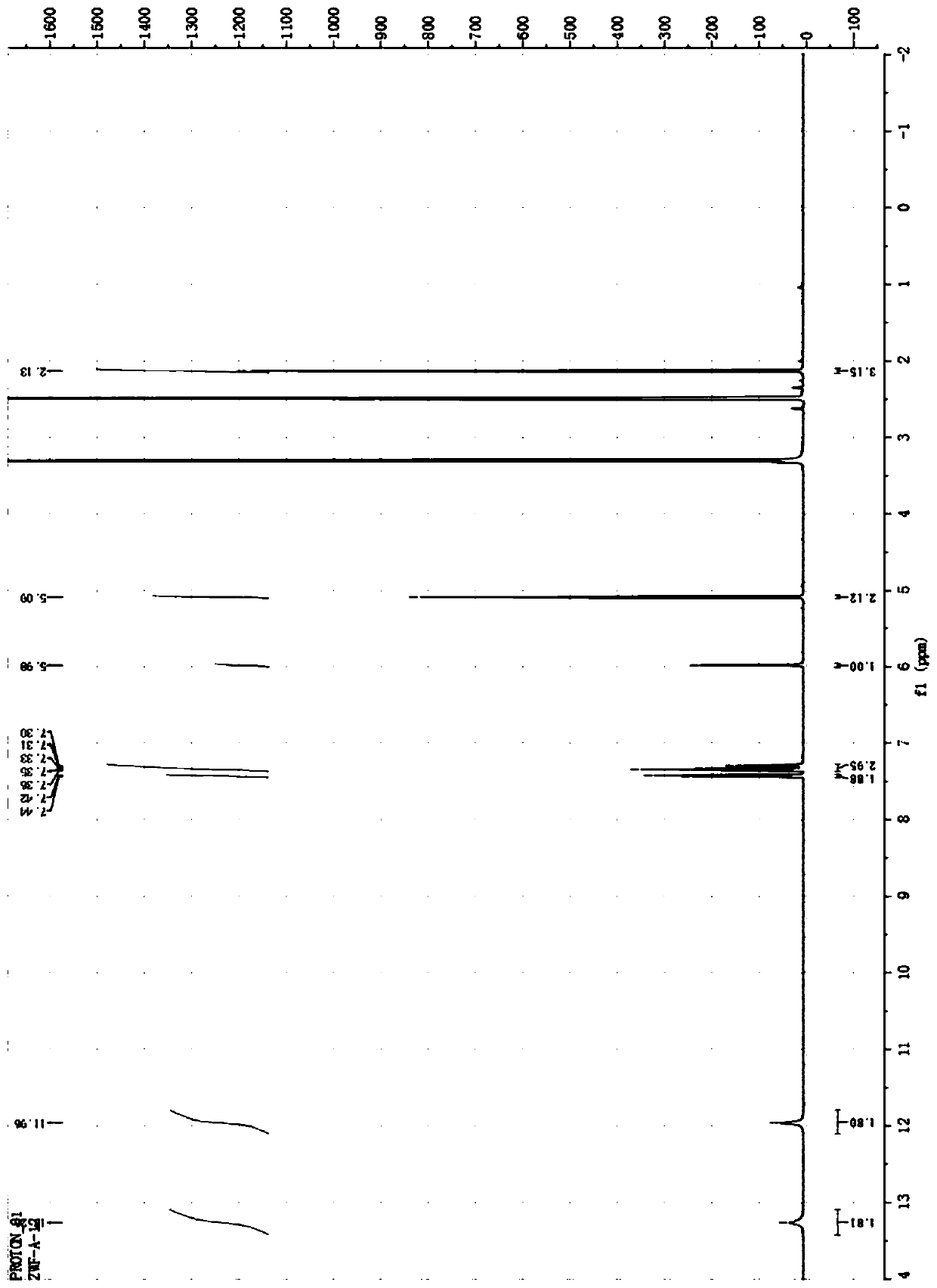
A kind of double 3,2-hydroxypyridone derivatives and preparation method and use thereof
A technology of hydroxypyridones and derivatives, which is applied in the direction of pharmaceutical formulations, drug combinations, and medical preparations containing active ingredients, etc., can solve problems such as toxic and side effects, and achieve the effect of low toxicity and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1) Preparation of HOPO-1-1
[0044] Dissolve 10 parts of diethyl oxalacetate sodium salt in 100 mL of tetrahydrofuran, then add 5 parts of chloroacetone, pass through ammonia gas for 4 hours, then slowly add 0.5 parts of aluminum chloride, and react for 5 days at room temperature. Stop the reaction, filter with suction, dissolve the filter cake in 700mL HCl, make the pH<3, stir for 0.5h, filter with suction, wash the filter cake with water, collect the filter cake, recrystallize with absolute ethanol, and dry the filter cake at 40°C to obtain Earth gray solid, yield: 31.2%.
[0045] 2) Preparation of HOPO-1-2
[0046] Add 10 parts of dichloromethane, 0.2 parts of benzyl bromide, and 0.2 parts of hexadecyl pyridinium chloride to an aqueous solution of 0.2 parts of the intermediate product HOPO-1-1 and 0.8 parts of potassium carbonate, and react at 40°C for 24 hours. Stop the reaction, separate the two phases, extract with dichloromethane, combine the organic phases, an...
Embodiment 2
[0056] 1) Preparation of HOPO-1-1
[0057] Dissolve 50 parts of diethyl oxalacetate sodium salt in 500 mL of tetrahydrofuran, then add 25 parts of chloroacetone, pass through ammonia gas for 4 hours, then slowly add 3.25 parts of aluminum chloride, and react for 5 days at room temperature. Stop the reaction, filter with suction, dissolve the filter cake in 700mL HCl, make the pH<3, stir for 1h, filter with suction, wash the filter cake with water, collect the filter cake, recrystallize with absolute ethanol, filter with suction, and dry at 40°C. An earthy gray solid was obtained, yield: 30.5%.
[0058] 2) Preparation of HOPO-1-2
[0059] Add 50mL of dichloromethane, 0.9 parts of benzyl bromide, and 0.9 parts of hexadecyl pyridinium chloride to an aqueous solution of 1 part of intermediate product HOPO-1-1 and 1 part of potassium carbonate, and react at 40°C for 24 hours. Stop the reaction, separate the two phases, extract with dichloromethane, combine the organic phases, and...
Embodiment 3
[0069] 1) Preparation of HOPO-1-1
[0070] Dissolve 100 parts of diethyl oxalacetate sodium salt in 1000 mL of tetrahydrofuran, then add 50 parts of chloroacetone, pass through ammonia gas for 4 hours, then slowly add 6.5 parts of aluminum chloride, and react for 5 days at room temperature. Stop the reaction, filter with suction, dissolve the filter cake in 700mL HCl, make the pH<3, stir for 1h, filter with suction, wash with water, recrystallize the filter cake with absolute ethanol, filter with suction, and dry at 40°C to obtain a gray solid. Yield: 30.9%.
[0071] 2) Preparation of HOPO-1-2
[0072] Add 100mL of dichloromethane, 1.8 parts of benzyl bromide, and 1.8 parts of hexadecyl pyridinium chloride to the aqueous solution of 2 parts of intermediate product HOPO-1-1 and 2 parts of potassium carbonate, and react at 40°C for 24h. Stop the reaction, separate the two phases, extract with dichloromethane, combine the organic phases, and rotary evaporate to obtain a light y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com