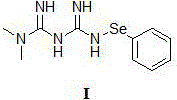Selenium-containing compound and use thereof
A compound and application technology, applied in the field of selenium-containing compounds and their preparation, can solve the problems of side effects, limited anti-cancer spectrum, limited compound structure types, etc., and achieve the effects of simple preparation method, high yield and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
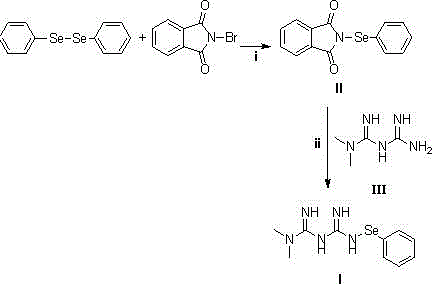
[0037] Embodiment 1: Synthesis of N-phenylselenide benzosuccinimide (compound of formula II)
[0038] Add N-bromobenzosuccinimide (147 mg, 1 mmol), diphenylselenide (312 mg, 1 mmol) and 10 ml of anhydrous acetonitrile into a three-necked flask, heat to 50 °C, and Under argon protection, after stirring for 24 hours, the crude product was obtained by distillation under reduced pressure, and column chromatography (mobile phase: ethyl acetate: petroleum ether = 2:1 (V:V)) gave 260 mg of a yellow powdery solid (compound of formula II) , yield 86%.
[0039] nuclear magnetic resonance 1 H NMR (400 MHz, CDCl 3 ) δ: 7.94-7.92 (2H, dd, J 1 = 5.5 Hz, J 2 = 3.0 Hz), 7.85-7.78(2H, dd, J 1 = 5.5 Hz, J 2 = 3.0 Hz), 7.64-7.60 (2H, m),7.52-7.46(1H, m), 7.32-7.30(2H, m). 13 C NMR: 176.2, 170.3, 140.4, 136.6, 134.5, 129.4, 124.3 123.7.
[0040] ESI-MS: m / z=325.2 (M + Na) + .
Embodiment 2
[0041] Embodiment 2: the synthesis of formula I compound
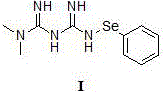
[0042] Add the compound of formula III (85 mg, 0.66 mmol), the compound of formula II N-phenylselenide substituted benzosuccinimide (200 mg, 0.66 mmol) and anhydrous DMF (10ml) in a three-necked flask, under argon After stirring overnight under protection, the solvent was distilled off under reduced pressure, and column chromatography (mobile phase: methanol: dichloromethane = 1:10) gave 210 mg of a white powder solid (compound of formula I), with a yield of 73%.
[0043] nuclear magnetic resonance 1 H NMR (DMSO) δ: 9.02 (1H, s), 7.84 (2H, s), 7.46-7.35 (2H, m), 7.28-7.13 (3H, m), 7.22 (1H, s), 2.88 (6H, s). 13 C NMR: 175.7, 158.3, 144.2, 130.2, 127.3, 124.9, 37.6.
[0044] ESI-MS: m / z=307.3 (M + Na) + .
[0045] in vitro pharmacology
experiment example 1
[0046] Experimental Example 1: Quinone Reductase Induction Experiment
[0047] Cell culture:
[0048] Mouse hepatoma cell line Hepa 1c1c7 was thawed and cultured at 37 o C, 5% CO 2 in a constant temperature incubator. The culture medium was α-MEM medium containing 10% fetal bovine serum and 1% antibiotics (100 U / mL penicillin, 100 μg / mL streptomycin). Replace the fresh culture medium every 2-3 days, and when the cell adhesion rate reaches above 85%, digest and count with 0.25% trypsin for later use.
[0049] Quinone reductase induction activity assay:
[0050] It has been known in the prior art that two-phase metabolic enzymes are of great significance for preventing the occurrence of tumors. As one of the two-phase metabolic enzymes, quinone reductase is a class of cell protection enzymes with detoxification and free radical scavenging functions. Paraquinone reductase Components with inductive activity are considered to have cancer-preventive effects at the tumor initiat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com