Pharmaceutical composition and application thereof
A composition and drug technology, applied in the field of tumor drug research, can solve the problems of no cancer treatment effect and poor pertinence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: Preparation of non-specifically expanded and activated T cells
[0074] The method for preparing non-specifically expanded and activated T cells comprises the following steps:
[0075] (1) Take 50 mL of peripheral blood from the patient (anticoagulated with heparin sodium), divide it into two 50 mL centrifuge tubes at 800 g, and centrifuge at 4°C for 15 min;
[0076] (2) After centrifugation, extract the upper layer of plasma and store it in a 50mL centrifuge tube in a refrigerator at 4°C;
[0077] (3) Dilute the blood cells in the centrifuge tube with normal saline 1:1, slowly add to the upper layer of the lymphocyte cell separation medium, and centrifuge at 800g, 20°C for 17min;
[0078] (4) After centrifugation, extract mononuclear cells (buffy coat) into a 50mL centrifuge tube, add normal saline to wash, centrifuge at 300g, 4°C for 8min, and wash twice;
[0079] (5) After cell counting, the cells were sorted by CD4 and CD8 magnetic beads, and the obtain...
Embodiment 2
[0083] Example 2: Inhibitory effect of pharmaceutical composition on mouse model renal cancer cells
[0084] (1) The establishment of the mouse kidney cancer model uses a subcutaneous inhibition model to facilitate the observation of tumor growth. Take 25 normal 6-week-old nude mice, each weighing about 20g, and inject 0.2mL renal cancer cell suspension subcutaneously at the right side of each nude mouse, and 10 days after the inoculation grows a 2-3mm tumor , the mouse melanoma model was successfully established.
[0085] (2) Grouping and processing
[0086] After modeling, mice were randomly divided into 5 groups, 5 in each group, including PBS control group (intraperitoneal injection of sterilized PBS 1mL), PD-1 monoclonal antibody treatment group (intraperitoneal injection of 2 mg / kg), and non-specific amplification and activation of T Cell treatment group (Bifidobacteria 5.83×10 9 ), the pharmaceutical composition treatment group, and the next day after the inoculation...
Embodiment 3
[0091] Embodiment 3: clinical experiment
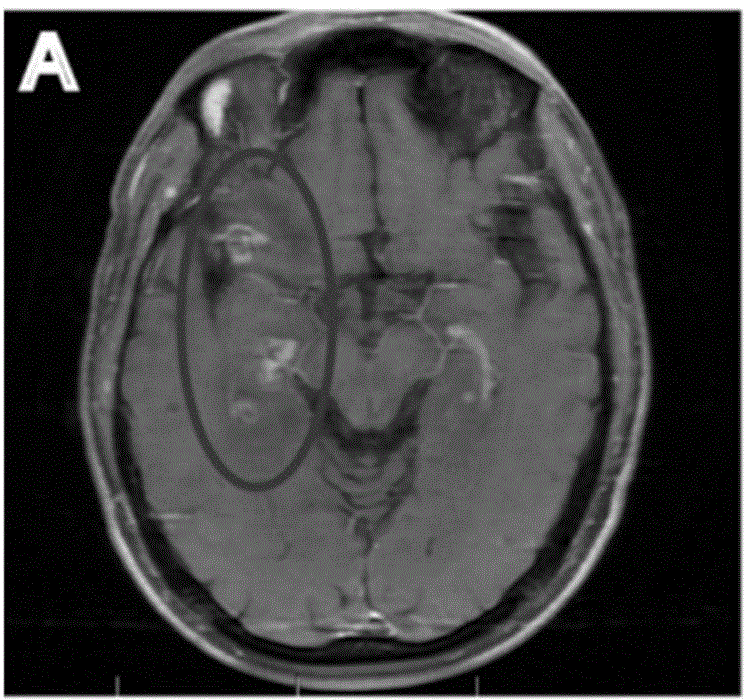
[0092] Male, 50 years old. In December 2012, he was diagnosed with clear cell carcinoma of the left kidney and metastases to the lung bone. After surgical resection of the left kidney cancer, bisphosphonates were used as maintenance therapy. In May 2013, he received γ-knife radiotherapy for brain metastases. 2013.9-2014.10, oral Sutent treatment due to lung tumor progression, and γ-knife radiotherapy and whole brain radiotherapy during and in 2013.11 due to new brain lesions. 2014.11-2015.5: Due to the progress of brain and lung metastases, the treatment was changed to everolimus, during which γ-knife stereotactic radiosurgery was performed in 2014.4.
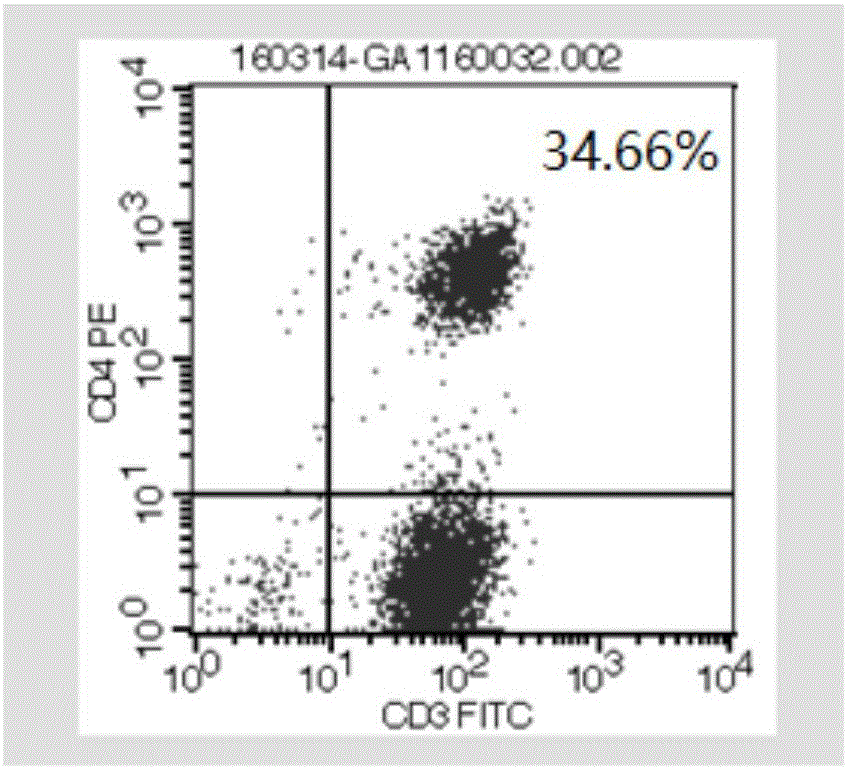
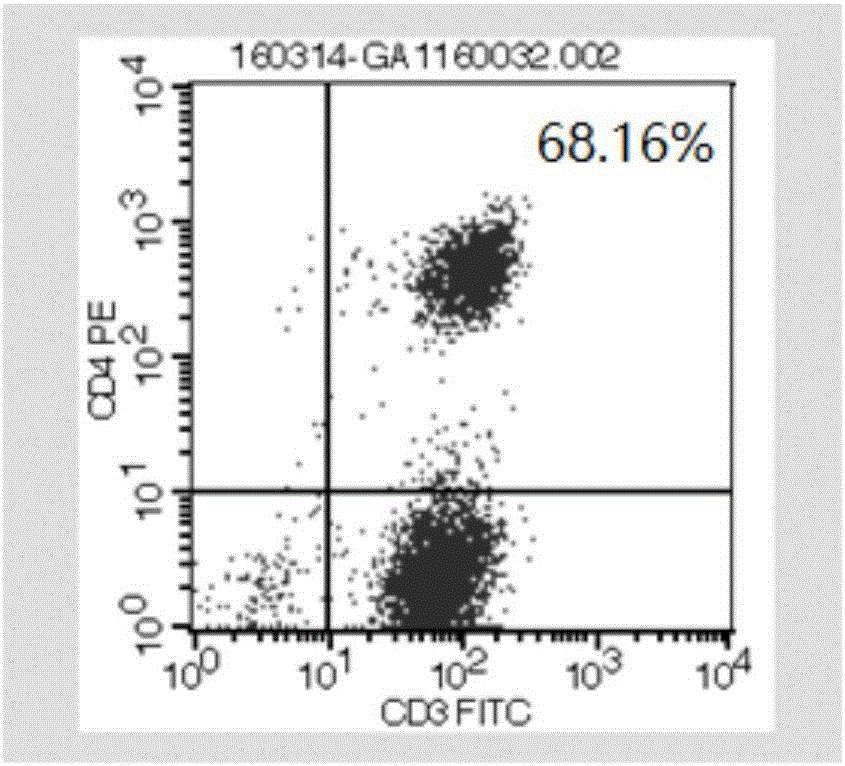
[0093] 2015.6-2016.1: Due to the re-progression of brain and chest tumors, PD-1 antibody (keytruda) 2mg / kg was applied once every three weeks, a total of 8 times. Non-specific expansion of activated T cells 5.83×10 9 / time, a total of 11 times. The results are shown in Figure 2. Fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com