Entecavir solid dispersion and Entecavir preparation
A technology of solid dispersion and entecavir, which is applied in the direction of pill delivery, antiviral agents, active ingredients of heterocyclic compounds, etc. It can solve the problems of easily destroying drug activity, difficult to crush and sieve, difficult to control the heating and cooling rate of melting method, etc. , to achieve the effect of improving bioavailability, improving solubility and dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
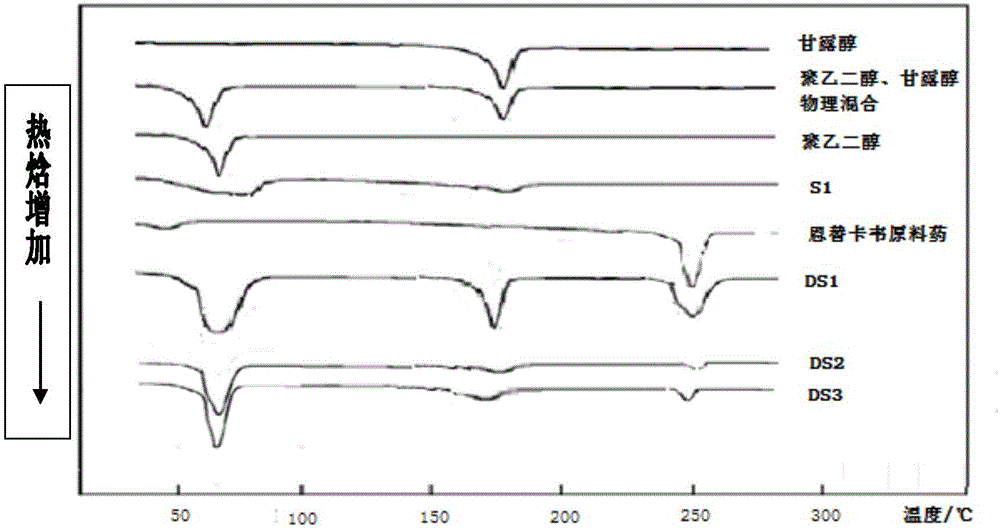
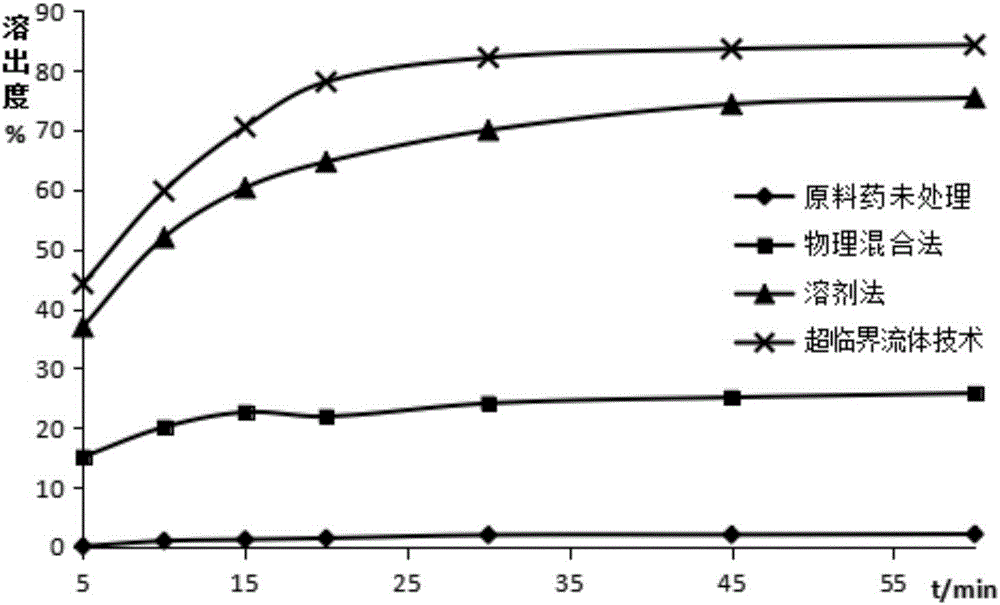
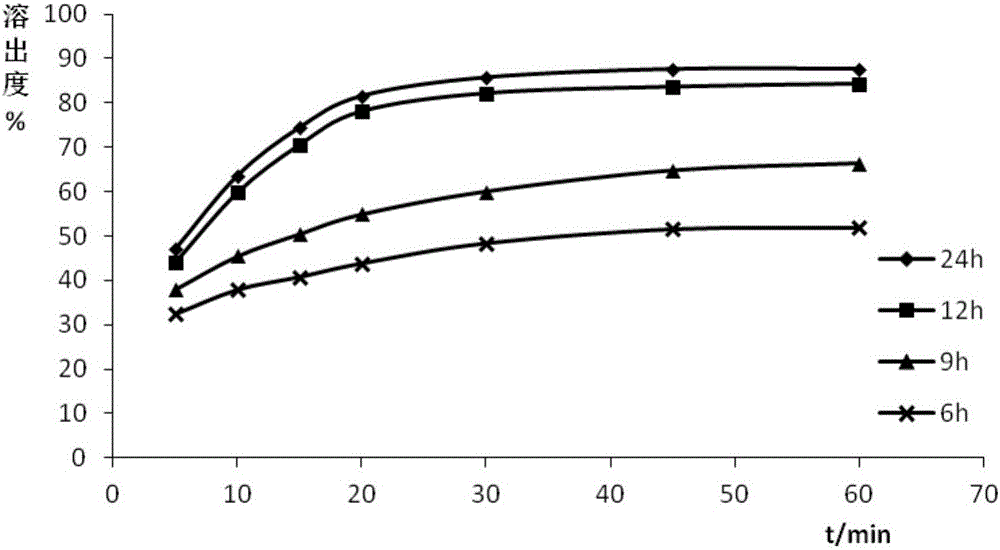
Image
Examples
Embodiment 1
[0074] This embodiment is used to illustrate the entecavir solid dispersion provided by the present invention.
[0075] Take 0.5g of entecavir, 2.0g of PEG-4000, and 0.5g of mannitol, mix them evenly, put them in an autoclave, set the preparation pressure to 25MPa, the temperature to 50°C, and the time to 12h. After the temperature in the kettle reaches the set value, into CO 2 Until the pressure reaches the set value, keep the temperature and pressure for the set time, collect the solid dispersion in the kettle under reduced pressure, pulverize, and sieve to obtain the entecavir solid dispersion of this embodiment, which is designated as S1.
Embodiment 2-5
[0083] The present examples 2-5 are used to illustrate the preferred process conditions for preparing entecavir solid dispersion by supercritical fluid technology provided by the present invention.
[0084] Using PEG as the hydrophilic carrier material, and the mass ratio of entecavir to PEG is 1:5, under the conditions of pressure of 25MPa and temperature of 50°C, the steps similar to those of Example 1 were used to prepare the samples of Example 2-5. Entecavir solid dispersion, the difference is that each embodiment sets the reaction time as 6h, 9h, 12h and 24h respectively, which is recorded as S2-S5.
Embodiment 6-11
[0086] The present examples 6-11 are used to illustrate the preferred process conditions for preparing entecavir solid dispersion by supercritical fluid technology provided by the present invention.
[0087] Under the conditions that the pressure is 25MPa, the temperature is 50°C, and the reaction time is 12h, the entecavir solid dispersions of Examples 2-5 are prepared by the steps similar to Example 1, except that the hydrophilic The dosage ratio of the carrier, entecavir and hydrophilic carrier is shown in the following table 1, and the obtained entecavir solid dispersion is recorded as S6-S11.
[0088] Table 1
[0089] sample carrier drug carrier ratio sample carrier drug carrier ratio S6 Mannitol 1:5 S9 PEG 1:10 S7 Mannitol 1:10 S10 PEG: Mannitol (4:1) 1:5 S8 PEG 1:5 S11 PEG: Mannitol (4:1) 1:10
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com