Method for producing freeze-dried preparation
A manufacturing method and drying technology, applied in freeze-drying conveying, powder conveying, inorganic inactive ingredients and other directions, can solve the problem of inaccurate content of active ingredients, and achieve the effect of accurate content of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
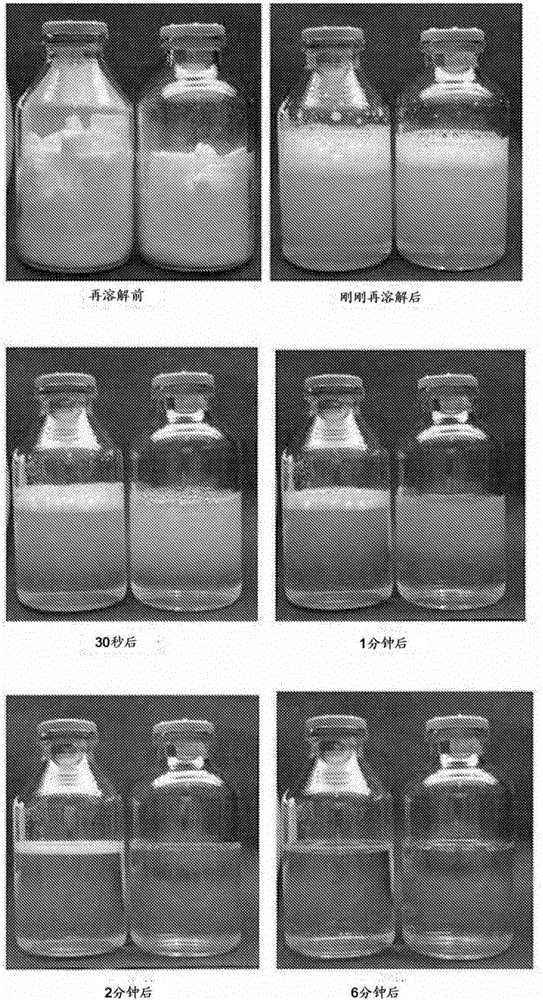
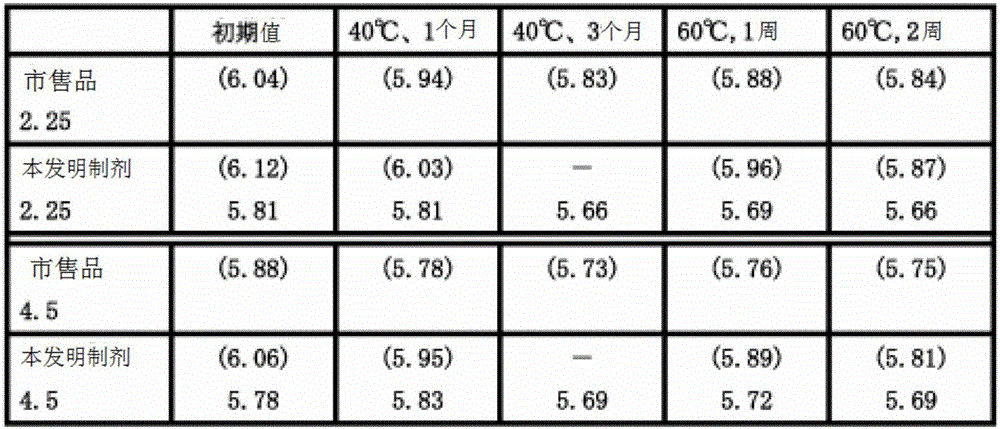
[0063] Dissolve 2.4 kg of sodium hydroxide in 56.25L of water for injection, blow carbon dioxide into it, and adjust the pH to about 7.7. To the sodium hydroxide aqueous solution bubbled with carbon dioxide, the pH was slightly adjusted to 7.7 with 0.5 M hydrochloric acid aqueous solution, and while nitrogen was bubbled, 2.976 kg of trizobactam and 23.81 kg of piperacillin hydrate were added, and then further A 0.1M aqueous hydrochloric acid solution and water for injection were added to obtain 125L of an aqueous solution with pH 6.5. The prepared solution was sterilized by filtration and dispensed into sterile containers, and then freeze-dried according to a conventional method to obtain 0.25 g (titer) of tazobactam sodium and 2.0 g of piperacillin sodium in each container ( Titer), or 0.5 g of trizobactam sodium (titer), and 4.0 g (titer) of piperacillin sodium.
[0064] Hereinafter, the preparation of the present invention containing 0.25 g of tazobactam sodium (titer) and 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com