D-fructose-6-phosphate aldolase a mutant, recombinant expression vector, genetically engineered bacteria and its application and reaction products
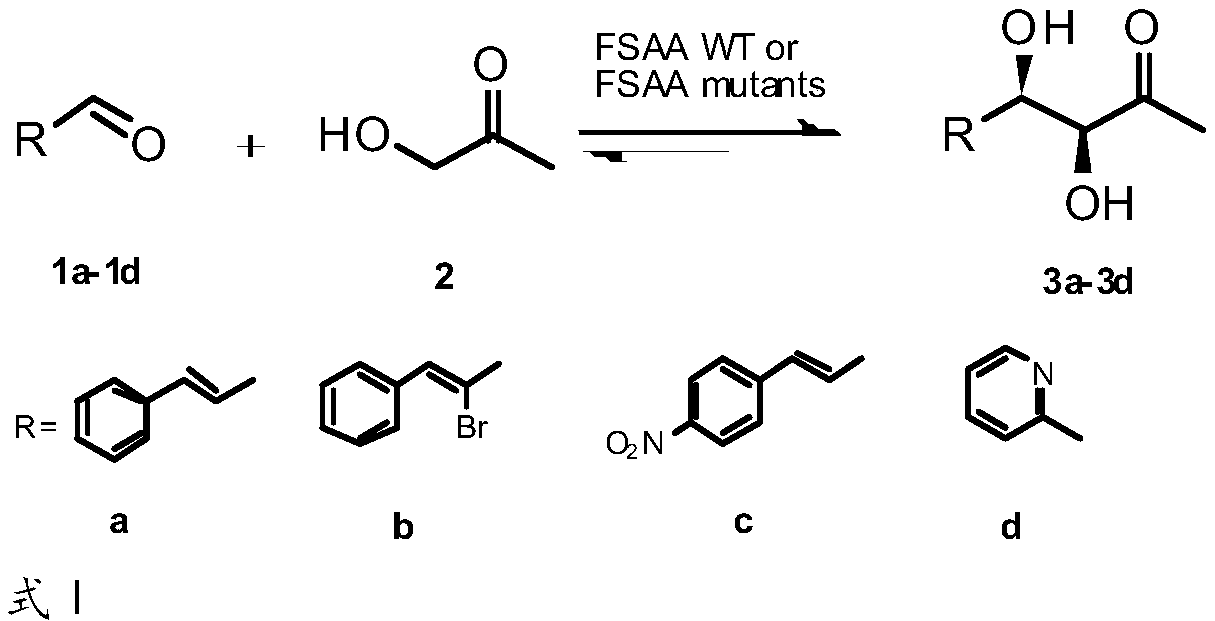
A phosphate aldolase and genetically engineered bacteria technology, applied in the fields of genetic engineering and enzyme engineering, can solve the problems that the activity cannot meet the requirements of industrialized production, reduce the reaction activity of cinnamaldehyde and replace the cinnamaldehyde, and achieve good industrial application development prospects, Efficient catalysis of asymmetric direct aldol condensation reaction, easy preparation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the construction of mutant
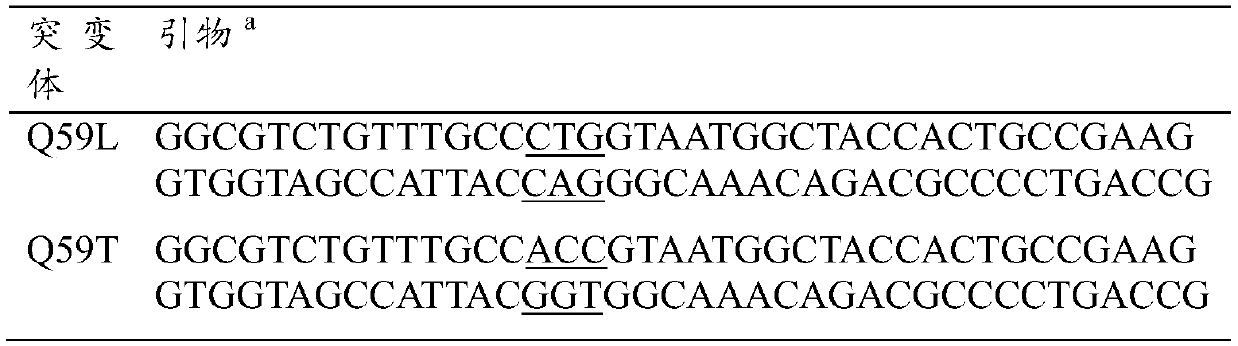
[0044] Using the oligonucleotide fragment containing the mutation point as a primer (Table 1), the recombinant plasmid pET-30a containing the FSAA gene was amplified by the QuickChangeTM method (Stratagene, La Jolla, CA).
[0045] Table 1 Mutant construction primers
[0046]
[0047] a Mutation sites are underlined
[0048] PCR reaction system: 5×PrimerSTAR buffer (Mg2+plus), 5 μL; dNTPs (2.5 mM each), 2.0 μL; upstream primer (10 μM), 1.0 μL; downstream primer (10 μM), 1.0 μL; recombinant plasmid template, 15 ng; PrimerSTARpolymeraseTM HS (2.5U / μL), 0.5 μL; add ddH2O to a total volume of 25 μL.
[0049] PCR program: (1) 98°C, 1min; (2) 98°C, 10s; (3) 55°C, 10s; (4) 72°C, 7min. Steps (2)-(4) were cycled 20 times and then cooled to 4°C.
[0050] After the PCR product is washed, it is digested with the restriction endonuclease DpnI that specifically recognizes the methylation site to degrade the template plasmid. Enzyme d...
Embodiment 2
[0052] Example 2: Induced expression of FSAA mutants
[0053] The engineered bacteria constructed in Example 1 were inoculated into LB liquid medium containing 50 μg / mL kanamycin and 20 μg / mL chloramphenicol, cultivated overnight at 37° C., and then transferred to In 100mL LB medium containing 50μg / mL kanamycin and 20μg / mL chloramphenicol, culture at 37°C and 220rpm until the cell concentration OD600 to about 0.6, add final concentration of 0.1mM IPTG and 1mg / mL L -Arabinose, after induction culture at 26°C for 7 hours, centrifuge at 4°C and 4000rpm for 10 minutes to collect the bacterial cells, and store at -80°C for future use.
Embodiment 3
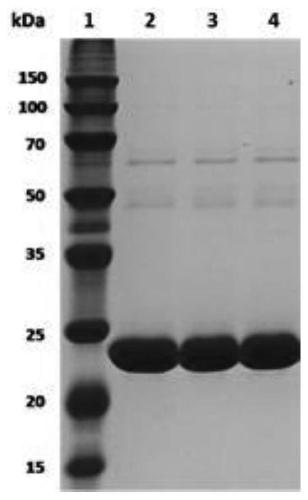
[0054] Embodiment 3: Separation and purification of FSAA mutant
[0055] The thalli cells that embodiment 2 collects are suspended in 20mL Na 2 HPO 4 -NaH 2 PO 4 In buffer solution (100mM, pH 8.0), shake well and then crush under ultrasonic wave (effective time 8min). The broken liquid was centrifuged at 12,000 rpm for 10 min to remove cell debris, and the supernatant (crude enzyme liquid) was collected for subsequent separation and purification of the enzyme. The purification column is a HisTrap (GE Healthcare) affinity chromatography column with a packing volume of 5 mL. First equilibrate the Ni-NTA column with a loading equilibration buffer (20 mM sodium phosphate, 500 mM NaCl and 20 mM imidazole, pH 7.4). Load crude enzyme solution at a rate of 1 / min, elute with loading equilibration buffer to remove unadsorbed protein, and finally collect target protein by eluting with elution buffer (20mM sodium phosphate, 500mM NaCl and 500mM imidazole, pH 7.4) . The enzyme soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com