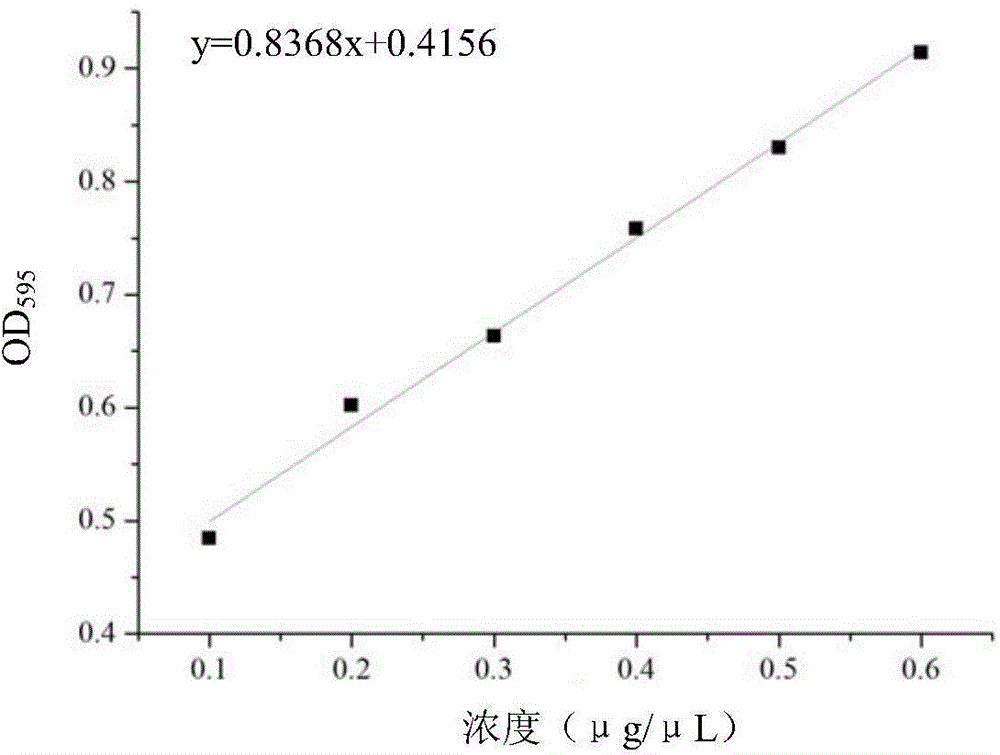
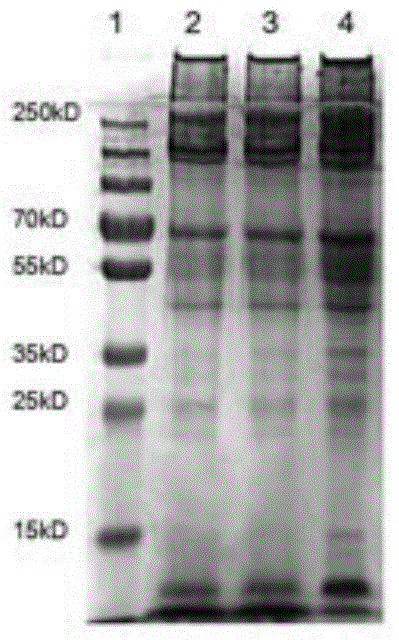
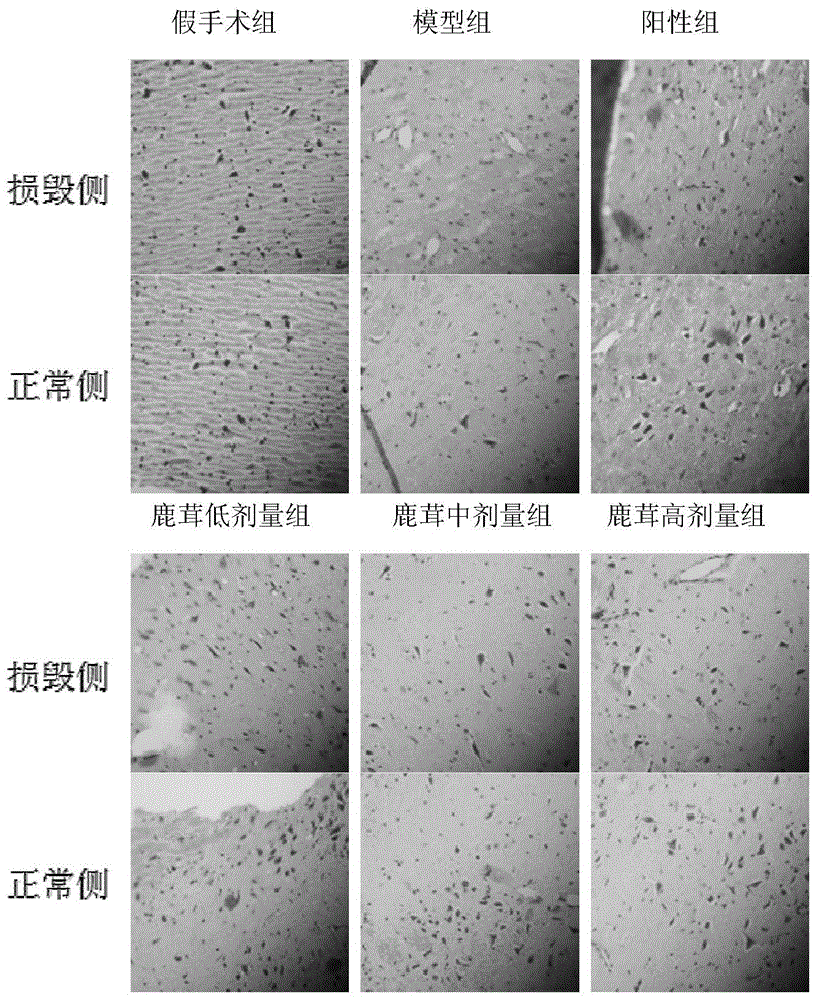
Application of pilose antler extract in preparation of products for preventing and/or treating Parkinson disease
A technology for Parkinson's disease and extract, which is applied in the application field of preparing products for preventing and/or treating Parkinson's disease, and can solve problems such as toxic side effects, little attention to non-motor symptoms, and inability to prevent dopaminergic neuron degeneration, etc. , to achieve a good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1, have the preparation of the velvet antler protein extract of preventing and / treating Parkinson's disease
[0078] 1. Preparation of velvet antler protein extract
[0079] The velvet protein extract is prepared according to the following method, and the velvet protein extract is the velvet protein extract for preventing and / or treating Parkinson's disease. details as follows:
[0080] The fresh velvet antler is directly crushed for the first time with a pulverizer to obtain the first crushed velvet antler. Put the velvet antler crushed for the first time into liquid nitrogen quick-freezing, put it into a superfine pulverizer, add an appropriate amount of water for the second crushing until it is minced meat, and obtain the second crushed velvet antler, the molecular particle size of the second crushed velvet antler is 75 μm. Add a certain amount of water to the second crushed velvet antler (the mass ratio of the second crushed velvet to water is 1:5), th...
Embodiment 2
[0161] Embodiment 2, the pharmacodynamic study of velvet antler protein extract
[0162] 1. Animal modeling
[0163] After intraperitoneal injection of 10% chloral hydrate according to the injection dose of 0.35mL / 100g body weight in rats was anesthetized, sensory loss was induced. Fix the head on the stereotaxic instrument, incise the scalp, remove the subcutaneous tissue and periosteum, and find the anterior and posterior fontanelles. It is determined that the right side of the substantia nigra compact part is 4.8mm posterior to the anterior bregma, 2.0mm to the right of the sagittal suture, and 8.0mm subperiosteum; the ventral tegmental area of the midbrain is 4.8mm posterior to the anterior bregma, 1.2mm to the right of the midline, and 8.0mm subperiosteal mm. After determining the coordinates, inject 8 μL of the prepared 6-OHDA solution into the substantia nigra pars compacta and the ventral tegmental area of the midbrain, and inject in divided doses, and keep the n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com