Oligosaccharide synthesizing method
A synthesis method and oligosaccharide technology, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of expensive catalysts, easy to pollute the environment, and low synthesis efficiency, and achieve cheap and easy-to-obtain catalysts, shorten The effect of simple reaction route and donor structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
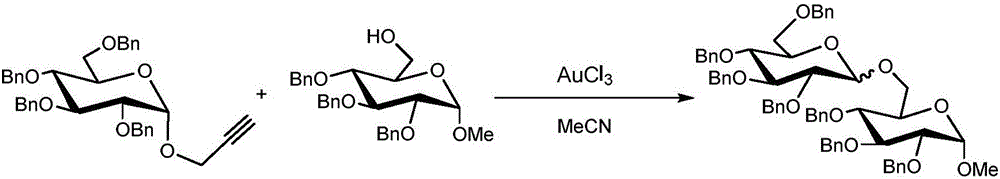
[0020] The present invention carries out the synthesis of oligosaccharide (3a) according to the following reaction equation:
[0021]
[0022] Among them: 1a is full benzyl protected galactosyl propargyl glycoside; 2a is 2,3,4-tri-O-benzoyl-6-O-trityl-α-D-glucoside; 3a is 2,3,4-Tri-O-benzoyl-6-O-(2,3,4,6-tetra-O-benzyl-D-galactosyl)-α-D-glucoside.
[0023] in N 2 Under protection, dry 29 mg (0.05 mmol) of all benzyl protected galactosyl propargyl glycosides with 37.5 mg (0.05 mmol) of 2,3,4-tri-O-benzoyl-6-O-trityl -α-D-glucoside was dissolved in 3ml of dichloromethane solvent, and 1.5mg of FeCl was added rapidly 3 ·6H 2 O into the reaction system, heated to 40°C for glycosylation reaction for 25 hours, TLC monitoring of the reaction process, after the completion of the reaction, add 200-300 mesh silica gel to the reaction solution, rotary evaporation, concentration to remove the solvent, and then separated by column chromatography , 45 mg of the product was oligosaccha...
Embodiment 2
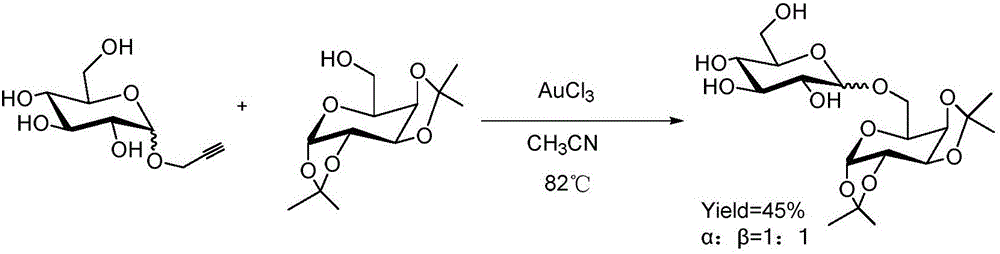
[0027] The present invention carries out the synthesis of oligosaccharide (3b) according to the following reaction equation:
[0028]
[0029] Among them: 1b is the whole benzyl protected glucose propargyl glycoside; 2b is 2,3,4-tri-O-benzoyl-6-hydroxy-α-D-glucoside; 3b is 2,3,4- Tris-O-benzoyl-6-O-(2,3,4,6-tetra-O-benzyl-D-glucosyl)-α-D-glucoside.
[0030] in N 2 Under protection, 0.06 mmol (35 mg) of dry whole benzyl-protected glucopropargyl glycoside and 0.05 mmol (25 mg) 2,3,4-tri-O-benzoyl-6-hydroxy-α-D-glucose methyl Glycosides and 1mg FeCl 3 Be dissolved in 3ml of acetonitrile solvent, be heated to 80 ℃ and carry out glycosylation reaction 15 hours, TLC monitors the reaction process, after the reaction is completed, reaction solution is by silica gel column chromatography (petroleum ether / ethyl acetate = 10:1) separation and purification, the product 43mg was oligosaccharide (3b), and its yield was 83%.
[0031] The product obtained above was analyzed and confirm...
Embodiment 3
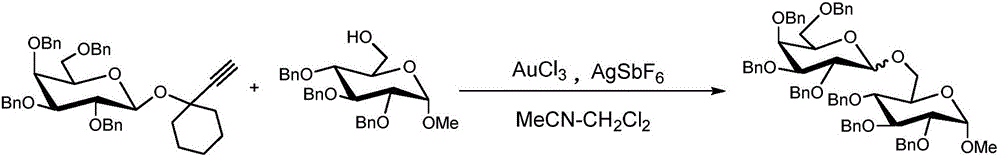
[0034] The present invention carries out the synthesis of oligosaccharide (3b) according to the following reaction equation:
[0035]
[0036] in N 2 Under protection, dry 0.1 mmol (58 mg) of whole benzyl protected glucosyl propargyl glycoside and 0.05 mmol (25 mg) of 2,3,4-tri-O-benzoyl-6-hydroxy-α-D-glucose methyl Glycosides and 10mg FeCl 3 / C(FeCl 3 The mass ratio to activated carbon is 1:1) dissolved in 1.5ml tetrahydrofuran solvent, heated to 80°C for glycosylation reaction for 8 hours, TLC to monitor the reaction progress, after the reaction was completed, the reaction solution was rotary evaporated, concentrated to remove the solvent, and passed through the silica gel column After separation and purification by chromatography (petroleum ether / ethyl acetate=10:1), 40 mg of the product was obtained as oligosaccharide (3b), and the yield was 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com