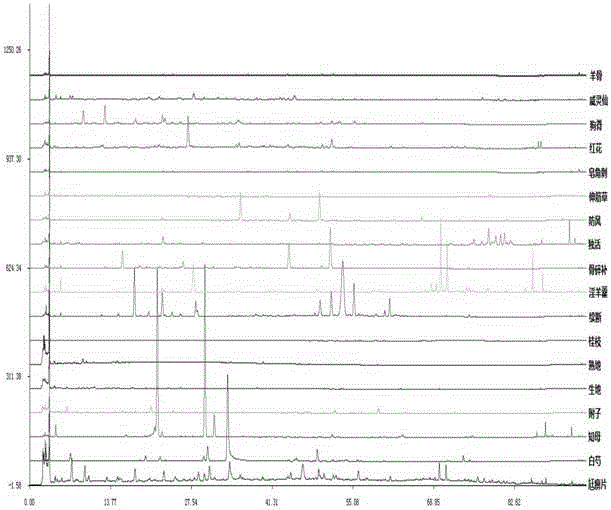
Method for establishing fingerprint of lame impediment treating pills and method for identifying lame impediment treating pills by using fingerprint method
A technology of fingerprint spectrum and establishment method, which is applied in the identification of Erbi tablets and the establishment of fingerprints of Erbi tablets, can solve the problems of complex effective chemical components, single chemical components cannot fully reflect the overall information, etc., and achieves good reproducibility, high precision effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] The present embodiment provides a method for establishing fingerprints of Miaobi Tablets, which includes:
[0076] Preparation of reference solution: Accurately weigh 5.01mg of icariin, place it in a 10ml measuring bottle, add methanol to dissolve, dilute to the mark, shake well; then accurately measure 1ml from it, place it in a 10ml measuring bottle, add Dissolve in methanol, dilute to the mark, and shake well to obtain a 0.05 mg / ml reference substance solution.
[0077] Preparation of the sample solution: get about 1.0 g of the Mubi Tablet sample (coating removed, finely ground), accurately weighed, add 25 ml of dilute ethanol solution to the Erlenmeyer flask with a stopper, weigh, and ultrasonically process (power 200W, Frequency 40kHz) 1h, take it out and let it cool, then weigh it, make up the lost weight with dilute ethanol, shake well, filter, get the filtrate to get the sample solution; prepare the sample solutions of each batch of Mianbi Tablets respectively. ...
Embodiment 2
[0085] The present embodiment provides a method for identifying Lubi Tablets by utilizing the fingerprints of Lubi Tablets obtained in Example 1, specifically:
[0086] (1) Take the sample to be tested, take the coating and grind it into powder, accurately weigh 1.0g, add 25ml of dilute ethanol solution to the conical flask with a stopper, weigh it, and ultrasonically treat it at a frequency of 40kHz for 60min, take it out and let it cool , weigh again, make up the lost weight with dilute ethanol, shake well, filter, and take the filtrate to obtain the sample solution to be tested;
[0087] (2) The sample solution to be tested is injected with 10 μl, using octadecylsilane bonded silica gel as a filler, and acetonitrile and 0.1% phosphoric acid aqueous solution to form a mobile phase, at a mobile phase flow rate of 1ml / min and a column temperature of 30 Under the condition of ℃, the gradient elution is carried out in the following manner, and the detection is carried out at the...
experiment example 1
[0094] Experimental example 1: Chromatographic conditions and system suitability test
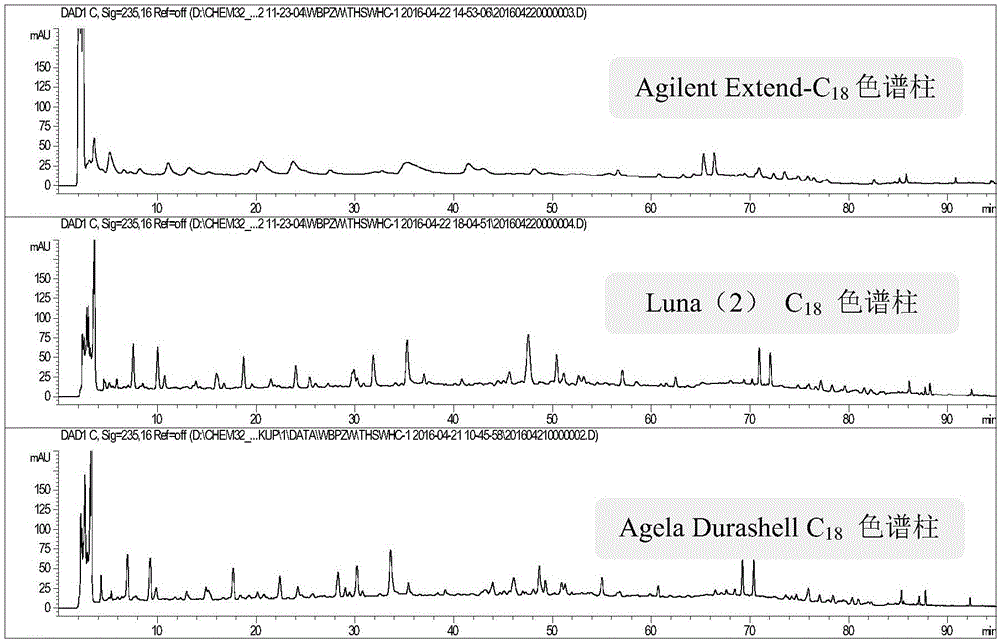
[0095] 1) Inspection of chromatographic column
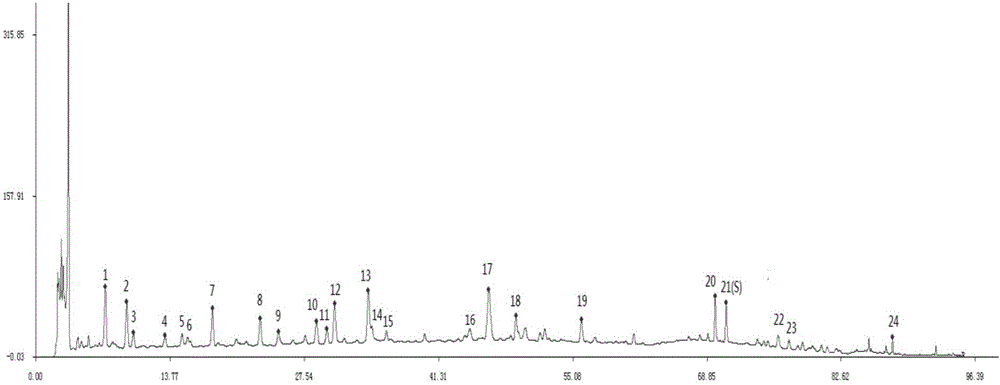
[0096] Selected Agilent Extend-C 18 (250×4.6mm, 5μm) chromatographic column, Agela Durashell C 18 (250×4.6mm, 5μm) column and Luna(2)C 18 (250×4.6mm, 5μm) chromatographic column Three kinds of chromatographic columns were investigated, and the results showed that Agela Durashell C 18 (250×4.6mm, 5μm) chromatographic column has better separation effect and peak shape, so AgelaDurashell C 18 (250×4.6mm, 5μm) chromatographic column. Chromatogram see figure 2 .
[0097] 2) Investigation of detection wavelength
[0098] Except that the detection wavelength conditions are other, the chromatographic detection steps are basically the same as those of the test product. By scanning the sample at a full wavelength of 190-600nm and comparing the isoabsorption diagrams, it was found that each peak can be well reflected at 235nm, and the baseline ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com