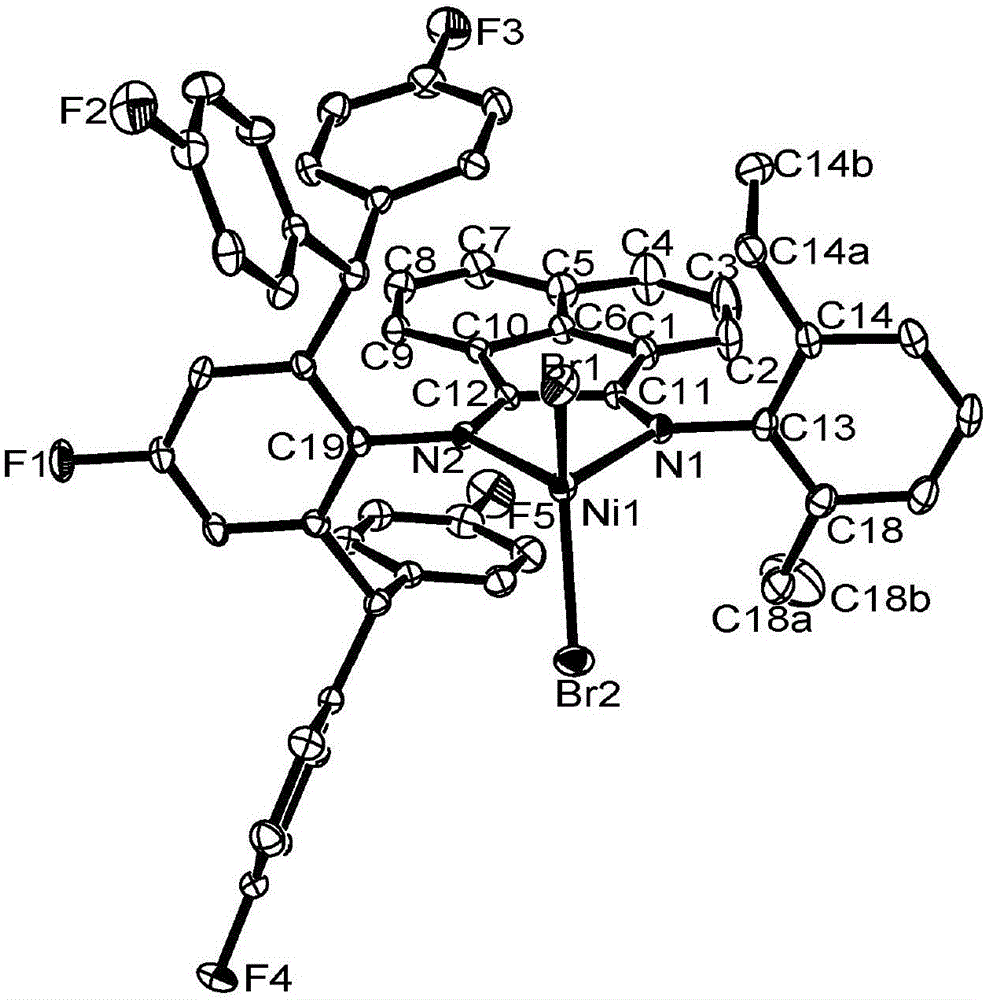
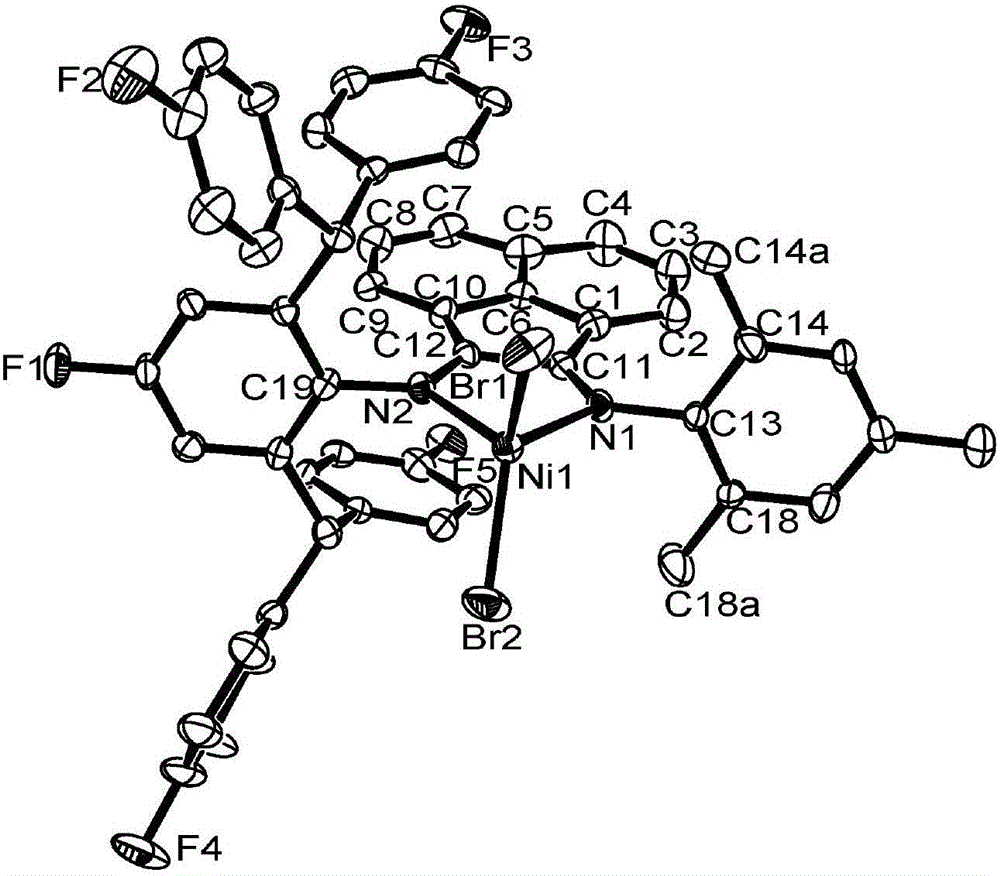
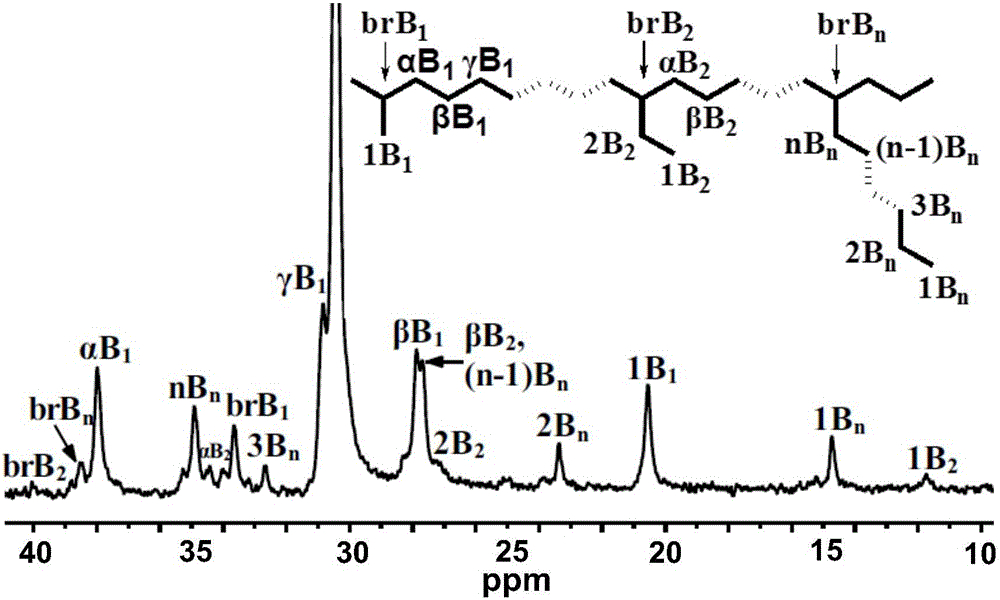
4,4'-difluorobenzhydryl containing asymmetric alpha-diimine nickel complex, and intermediate, preparation method and application thereof
A technology of difluorobenzhydryl and nickel complexes, which is applied in the preparation of imino compounds, compounds containing elements of group 8/9/10/18 of the periodic table, nickel organic compounds, etc., which can solve the problem of not being able to prepare high molecular weight Polyethylene elastomer material and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] 2-(2,6-bis(4,4'-difluorobenzhydryl)-4-fluoroanilino)acenaphthylenone represented by formula (V) was prepared.
[0127] Add the amount of catalyst (1.25g) of p-toluenesulfonic acid was refluxed for 5h. The solvent was removed, and the residue was subjected to basic alumina column chromatography with a mixed solvent of ethyl acetate and petroleum ether at a volume ratio of 1:50, and the eluted fraction was detected through a thin-layer silica gel plate, and the developing solvent was petroleum ether and ethyl acetate The volume ratio of the ester was a mixed solvent of 10:1, the third fraction was collected, and an orange-yellow solid was obtained after removing the solvent. Yield: 67%. Melting point: 181-183°C.
[0128] The structural confirmation data are as follows:
[0129] FT-IR (KBr, cm -1 ):3025.9(w), 1722.6(m), 1649.9(m), 1595.3(m), 1491.5(m), 1446.6(m), 1274.4(w), 1026.4(m), 694.7(vs).
[0130] 1 H NMR (600MHz, CDCl 3 ,TMS):δ8.11(d,J=8.1Hz,1H,An–H),8.08(d...
Embodiment 2
[0135] 1-(2,6-dimethylaniline)-2-(2,6-bis(4,4'-difluorobenzhydryl)-4-fluoroaniline) acenaphthene[ L1], where R 1 is methyl, R 2 for hydrogen.
[0136] 2-(2,6-bis(4,4'-difluorobenzhydryl)-4-fluoroaniline)acenaphthylenone (1.36g, 2.0mmol) and 2,6-dimethylaniline (0.36g, 3.0 mmol) in toluene (100 mL) was added a catalytic amount of p-toluenesulfonic acid, heated to reflux for 10 h. The solvent toluene was removed, and the residue was subjected to basic alumina column chromatography with a mixed solvent of ethyl acetate and petroleum ether at a volume ratio of 1:50. The eluted fractions were examined through a thin-layer silica gel plate, the second fraction was collected, and the solvent was removed to give an orange-yellow solid. Yield: 56%. Melting point: 197-199°C.
[0137] The structural confirmation data are as follows:
[0138] FT-IR (KBr, cm -1 ):3048(w),2924(w),1670(m),1642(m),1598(s),1505(vs),1224(vs),1157(s),1096(m),830(s ), 773(s).
[0139] 1 H NMR (600MHz, ...
Embodiment 3
[0144] 1-(2,6-diethylaniline)-2-(2,6-bis(4,4'-difluorobenzhydryl)-4-fluoroaniline) acenaphthene[ L2], where R 1 is ethyl, R 2 for hydrogen.
[0145] 2-(2,6-bis(4,4'-difluorobenzhydryl)-4-fluoroaniline)acenaphthylenone (1.00g, 1.47mmol) and 2,6-diethylaniline (0.33g, 2.21 mmol) in toluene (100 mL) was added a catalytic amount of p-toluenesulfonic acid, heated to reflux for 10 h. The solvent toluene was removed, and the residue was subjected to basic alumina column chromatography with a mixed solvent of ethyl acetate and petroleum ether at a volume ratio of 1:50. The eluted fractions were examined through a thin-layer silica gel plate, the second fraction was collected, and the solvent was removed to give an orange-yellow solid. Yield: 39%. Melting point: 227-229°C.
[0146] The structural confirmation data are as follows:
[0147] FT-IR (KBr, cm -1 ):3057(w), 2968(w), 2931(w), 1662(m), 1639(m), 1597(s), 1505(vs), 1456(s), 1439(s), 1221(vs ), 1158(s), 1099(m), 830(s), 7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com